In this issue of Blood, Oskarsdottir et al investigate a method for monitoring warfarin therapy with key improvements over the international normalized ratio (INR).1
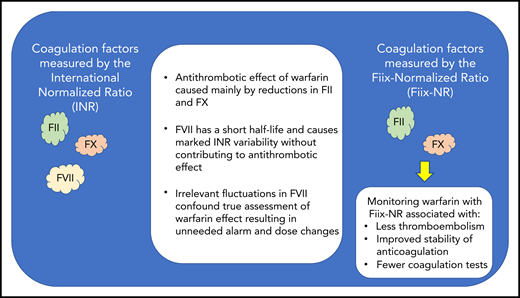
Key differences between the INR and the Fiix-Normalized Ratio (Fiix-NR).
Key differences between the INR and the Fiix-Normalized Ratio (Fiix-NR).
The INR has been the recognized standard for laboratory monitoring of warfarin therapy for decades.2 Although the INR is an improvement over the prothrombin time (PT) ratio, many patients receiving warfarin therapy fail to achieve adequate INR control. The findings of Oskarsdottir et al indicate that the INR test itself may be 1 reason underlying this ubiquitous problem.
The PT, which is used to calculate the INR, responds to warfarin-induced reductions in the activity of 3 of the 4 vitamin K–dependent procoagulant clotting factors (ie, factor II [FII], FVII, and FX) at a rate proportional to their respective half-lives (see figure).2 The actual antithrombotic effect of warfarin corresponds to reductions in the activity of FII and FX, but not FVII. However, with a short half-life of 4 to 6 hours, FVII may be responsible for INR fluctuations that cause false alarm and lead to unnecessary warfarin dose changes whereas both FII and FX with their much longer half-lives and their important antithrombotic effect remain relatively unaffected.1 Based on this observation, Oskarsdottir et al have introduced a new PT-based test called the Fiix-PT (pronounced “Fix-PT”) from which a normalized ratio (Fiix-NR) is calculated that is responsive only to reductions in the activity of FII and FX. The efficacy of Fiix-NR compared with INR monitoring of warfarin has been previously demonstrated by this same research team in a small randomized controlled trial, in which Fiix-NR monitoring was associated with reduced anticoagulation variability, fewer warfarin dose changes, and half the rate of thromboembolic events.3
In this issue, the effectiveness of replacing warfarin INR monitoring with Fiix-NR monitoring at a single center in Iceland is reported using an interrupted time series study design.1 The results of the earlier randomized controlled trials were largely confirmed in this real-world observational study with reductions in thromboembolic events (relative risk, 0.45; 95% confidence interval, 0.25-0.75), a 24% reduction in the number of tests, and a 34% reduction in the number of warfarin dose changes per patient per year. Differences in major bleeding (MB) events between INR- and Fiix-NR–monitored patients were not significant.
The linkage between stability of warfarin therapy and patient-important outcomes like thromboembolic events, MB, and mortality is well established.4 A key criticism of clinical trials evaluating the efficacy of direct oral anticoagulants (DOACs) was that the achieved stability of warfarin therapy in the comparator arms was less than ideal and varied widely between countries enrolling patients in these trials.5 The stability of warfarin therapy is often measured in terms of the percentage of time spent in the targeted INR range and INR variability.4 Many studies have explored interventions to improve the stability of warfarin therapy including the use of specialized anticoagulation management services (AMS) and the use of warfarin-dosing algorithms. It is important to note that the outcome improvements reported by Oskarsdottir et al occurred in a specialized AMS that was already using validated computerized warfarin-dosing algorithms. Although these types of interventions have been associated with increased INR stability, their implementation requires significant training and coordination of effort, and uptake has been variable across health systems. In contrast, implementing Fiix-NR monitoring should be less complicated and require less effort as it can be easily measured manually or on any semiautomatic laboratory system.1 The feasibility of implementing Fiix-NR monitoring in a health system has been demonstrated by Oskarsdottir et al as all INR monitoring of warfarin has been replaced by the Fiix-NR in their system since 2016.1
As efficacy of the Fiix-NR has been demonstrated in a randomized clinical trial3 and effectiveness demonstrated in a real-world observational study,1 it now seems that more widespread implementation of Fiix-NR warfarin monitoring should become a high priority. Several implementation science frameworks exist to guide further research aimed at expanding this potentially groundbreaking intervention to additional health systems.6 In particular, hybrid study designs focused on testing Fiix-NR implementation strategies while observing and gathering further information on the clinical outcomes associated with this intervention should be explored.7
A potential limitation of the Fiix-NR is that it requires patients to travel to laboratories for blood samples collected via venipuncture. Many AMSs and laboratories use fingerstick INRs obtained from point-of-care INR monitors. Some patients even use these portable monitors to measure their own INR at home. However, if clinical outcomes associated with the Fiix-NR are confirmed after wider implementation, it seems reasonable that point-of-care monitors could be configured to report the Fiix-NR instead of the INR.
For many patients, DOACs will be preferred over warfarin as they do not require routine coagulation monitoring and have been associated with improved clinical outcomes, in particular decreased risk of intracranial bleeding.8 However, DOACs have been shown to be either harmful or ineffective in patients with mechanical heart valves, left ventricular thrombi, and antiphospholipid antibody syndrome. Other patients taking certain interacting medications (eg, phenytoin, carbamazepine, rifampin) are required to take warfarin therapy because, unlike warfarin, the anticoagulant effect of DOACs cannot be easily monitored and titrated. Warfarin is also recommended for stroke prevention in patients with atrial fibrillation and moderate to severe mitral stenosis.9 Thus, for patients requiring warfarin therapy, a better monitoring method associated with improved stability and reduced thromboembolic risk like the Fiix-NR is an important advance.
Conflict-of-interest disclosure: D.M.W. has received research funding and consulting fees from Roche Diagnostics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal