Abstract
Cyclic thrombocytopenia (CTP) is a rare disease, which is characterized by periodic fluctuation of the platelet count. The pathogenesis of CTP is unknown and most likely heterogeneous. Patients with CTP are almost always misdiagnosed as having primary immune thrombocytopenia (ITP). The interval between ITP and CTP diagnosis can be many years. CTP patients often receive ITP-specific therapies including corticosteroids, thrombopoietin receptor agonists, rituximab, and splenectomy, which are followed by a transient increase in platelet count that is wrongly attributed to treatment effect with inevitable “relapse.” CTP can be diagnosed by frequent platelet count monitoring, which reveals a typical pattern of periodic platelet cycling. An early diagnosis of CTP will prevent these patients from being exposed to possibly harmful therapies. The bleeding phenotype is usually mild and consists of mucocutaneous bleeding at the time when the platelet count is at its nadir. Severe bleeding from other sites can occur but is rare. Some patients respond to cyclosporine A or to danazol, but most patients do not respond to any therapy. CTP can be associated with hematological malignancies or disorders of the thyroid gland. Nevertheless, spontaneous remissions can occur, even after many years.
Introduction
Cyclic thrombocytopenia (CTP) is characterized by periodic platelet count fluctuations at intervals of a few weeks. Platelet count may be as low as <5 × 109/L and is followed by a rapid increase to normal or even supernormal levels. Cycle duration varies individually and usually ranges between 14 and 36 days, potentially reflecting different etiologies. Consequently, platelet nadir can be associated with bleeding, which is usually mild or moderate, and platelet zenith can induce a hypercoagulable state. Initially, most CTP patients are typically misdiagnosed as having primary immune thrombocytopenia (ITP). The diagnosis of CTP is usually established, if at all, much later, often at a time when ITP-specific therapies have failed.
During the last century, only a limited number of CTP patients has been reported. In a registry from Canada, for instance, 269 of 614 patients (44%) who were referred to a tertiary hematology clinic for a platelet count of <150 × 109/L were classified as having ITP, but only 4 (0.7%) as having CTP.1 CTP is undoubtedly rare but certainly must not be neglected.
With this article, we want to (1) raise awareness about CTP as 1 cause of isolated thrombocytopenia, particularly in patients with an inadequate response to ITP therapy; (2) outline diagnostic strategies that might help in identifying these patients at the earliest stage; and (3) discuss the clinical course of these patients and the ensuing management strategies. CTP can occur in adults and children alike. This article focuses on adult patients and not all of our assertions may be applicable to juveniles.
Pathogenesis
The pathogenesis of CTP is unclear. Laboratory and clinical features that have been observed in CTP patients are generally heterogeneous, not mutually exclusive, and comprise accelerated platelet destruction, bone marrow abnormalities, T-cell receptor rearrangement, impaired regulatory mechanisms of platelet production, a connection with other disorders, and synchrony with the menstrual cycle (Table 1 2-35 ). Whether these conditions are causative, transient, reactive, a byproduct of as-yet-undiscovered pathomechanisms, or coincidental is unknown. They are addressed in some detail because they may provide important clues for the identification of CTP patients.
Pathogenesis of CTP
| . | Patients, n . | References . |
|---|---|---|
| (Auto)immune-mediated platelet destruction | 16 | 2-15 |
| Megakaryocytic hypoplasia | 15 | 4, 9, 11, 12, 14, 16-21 |
| T-cell receptor rearrangement | 7 | 14, 15, 22 |
| Abnormal regulation of platelet production | 12 | 10, 19, 21, 23-30 |
| Menstrual cycle synchronized | 10 | 2, 3, 7, 15, 19, 31-33 |
| Associated with other disorders | ||
| Disorders of the thyroid gland | 10 | 2, 15, 27 |
| Hematological malignancies | 22 | 14, 15, 21, 25, 26, 34 |
| Cyclic neutropenia | 1 | 35 |
| . | Patients, n . | References . |
|---|---|---|
| (Auto)immune-mediated platelet destruction | 16 | 2-15 |
| Megakaryocytic hypoplasia | 15 | 4, 9, 11, 12, 14, 16-21 |
| T-cell receptor rearrangement | 7 | 14, 15, 22 |
| Abnormal regulation of platelet production | 12 | 10, 19, 21, 23-30 |
| Menstrual cycle synchronized | 10 | 2, 3, 7, 15, 19, 31-33 |
| Associated with other disorders | ||
| Disorders of the thyroid gland | 10 | 2, 15, 27 |
| Hematological malignancies | 22 | 14, 15, 21, 25, 26, 34 |
| Cyclic neutropenia | 1 | 35 |
Proposed pathomechanisms are heterogeneous and not necessarily mutually exclusive.
(Auto)immune-mediated platelet destruction
Presence of antiplatelet antibodies has been described in a relatively large number of CTP patients.2-15 In a patient from Japan, for instance, an inverse relationship between platelet count and levels of immunoglobulin G (IgG) antibodies directed against glycoprotein IIIa was detected in the absence of any bone marrow abnormalities.7 Menitove and coworkers reported 2 patients in whom platelet-associated IgG varied inversely with the platelet count.5 In one of them, IgG on the platelet surface was directed against glycoprotein IIb/IIIa, was high at all cycle times, and was highest when the platelet count was low.
Megakaryocytic hypoplasia
Balduini and coworkers described a patient with cyclic disappearance of mature and immature megakaryocytes.16 Later, the patient developed permanent amegakaryocytosis and finally died of intracranial bleeding. Several other CTP patients with abnormal megakaryocytopoiesis have been reported.4,9,11,12,14,17-21
T-cell receptor rearrangement
In 2002, we found a clonal T-cell receptor rearrangement in a patient with CTP, suggesting a clonal T-cell–mediated process to be involved in the pathology of the disease. Reticulocyte and neutrophil counts fluctuated in parallel with the platelet count.22 We later identified a clonal T-cell receptor rearrangement in 5 additional CTP patients.15 Fogarty and coworkers described a patient with T-cell large granular lymphocytic leukemia (T-LGL) who had a T-cell receptor rearrangement and, in addition, antiplatelet antibodies that were highest just prior to the platelet nadir.14
Abnormal regulation of platelet production
In 2 of our patients, platelet counts were inversely correlated with thrombopoietin (TPO) levels, suggesting defective regulation of platelet production, an observation also made by several other investigators.15 In a patient with CTP and inversely fluctuating TPO levels, Zhang and coworkers identified a heterozygous MPL c.1210G>A mutation, which led to a p.Gly404Arg substitution. The mutant failed to support TPO-stimulated growth of Ba/F3 cells that express wild-type MPL and otherwise exhibit interleukin-3–independent growth in response to TPO.30
Menstrual cycle synchronized
There is a female propensity in CTP and the disease sometimes occurs in synchrony with the menstrual cycle with the zenith typically in the middle of menstrual cycles. Chen and coworkers described a woman with menstrual cycle–synchronized CTP in whom extensive laboratory workup, including bone marrow biopsy and TPO measurements, was uninformative.33 We recently identified a woman with CTP in whom the platelet nadir was at the time of menstrual bleeding. Laboratory testing revealed both a T-cell receptor rearrangement as well as antiplatelet antibodies and she had hypothyroidism.15
Associated with other disorders
Eight of our 9 CTP patients, all female, had a disorder of the thyroid gland.15 Thyrotoxicosis and chronic thyroiditis were also found in 1 patient with CTP each by Brey and coworkers and by Yujiri and coworkers.2,27 The mechanisms behind this striking association remain obscure. Interestingly, a strong association between autoimmune thyroid diseases and ITP is also well documented.36
A connection between CTP and hematological malignancies has long been speculated. Platelet oscillations were observed in as many as 18 patients with polycythemia vera (PCV) who were treated with hydroxyurea and in 1 PCV patient not receiving myelosuppression.21,25,26,34 Two CTP patients described by Steinbrecher and coworkers developed acute myeloid leukemia and T-LGL, respectively.15
Recently, Yabushita and coworkers reported an adult patient with CTP who also had periodic cycling of neutrophils.35 Because fluctuating anti-neutrophil antibodies were detected, the diagnosis of concomitant primary cyclic immune neutropenia was established. Cycling neutropenia (CyN) is a rare disease and, to a certain extent, resembles CTP in terms of periodic fluctuation of blood cells. In patients with CyN, a periodic decrease in the circulating neutrophil numbers from normal values to very low values occurs and the period is typically in the range of 19 to 21 days.37 There are several important differences between CyN and CTP. CyN occurs primarily in children and is attributable to mutations in the gene for the enzyme neutrophil elastase, ELANE.38 In contrast, the prevalence of CTP is higher in the adult population and a relationship between molecular defects and CTP is not obvious. CTP occurs more often in women than in men whereas there is no gender difference in CyN. Obviously, patients with CyN have infections at the time of neutrophil nadir whereas patients with CTP suffer from mucocutaneous bleeding at the time of thrombocytopenia. Granulocyte colony-stimulating factor shortens the duration of neutropenia and can prevent infections. TPO-receptor agonists (TPO-RAs) do not affect the duration of thrombocytopenia and have, therefore, no effect on bleeding.39
Diagnosis
Patient 1
Patient 1 is a 51-year-old otherwise healthy man who was referred to our hospital because of mucocutaneous bleeding and a platelet count of 2 × 109/L. During the preceding months, he had noticed small hematomas on the forearms and occasional mild nose and gum bleeding. All other routine blood tests were normal. Antibodies against glycoprotein IIb/IIIa and glycoprotein Ib/IX were detected. The diagnosis of ITP was made, and he was treated with prednisolone (1.5 mg/kg body weight per day; reduction to 50 mg per day after 7 days) and intravenous immunoglobulin (IVIG) at a dose of 1 g/kg of body weight on 2 consecutive days. The platelet count increased to 356 × 109/L within 10 days, and bleeding ceased. Platelets, however, dropped to 137 × 109/L after 1 week and to 4 × 109/L after 2 weeks. The dose of prednisolone was slowly tapered to 12.5 mg per day and TPO-RA treatment with eltrombopag (50 mg per day) was started. The platelet count was monitored at regular intervals. As depicted in Figure 1, platelet counts exceeding the upper normal range were followed by platelet counts ranging between ∼50 × 109/L and 200 × 109/L. On 1 occasion, 3 weeks after eltrombopag initiation, severe thrombocytopenia with a platelet count of <10 × 109/L and moderate mucocutaneous bleeding was encountered. After 10 weeks, the dose of eltrombopag was reduced to 25 mg daily because of a platelet count of 578 × 109/L. Consequently, severe thrombocytopenia (9 × 109/L) recurred, this time without bleeding.
Proposed algorithm to distinguish between ITP and CTP at an early stage. After failure of corticosteroids and a TPO receptor agonist (TPO-RA), consider clinical and laboratory features listed in Table 2. Provided that the bleeding phenotype is mild, treatment is suspended, and the blood count is monitored at regular intervals over a period of several weeks. The finding of a periodic platelet count fluctuation is indicative for CTP.
Proposed algorithm to distinguish between ITP and CTP at an early stage. After failure of corticosteroids and a TPO receptor agonist (TPO-RA), consider clinical and laboratory features listed in Table 2. Provided that the bleeding phenotype is mild, treatment is suspended, and the blood count is monitored at regular intervals over a period of several weeks. The finding of a periodic platelet count fluctuation is indicative for CTP.
Comment
Mucocutaneous bleeding together with a low platelet count in the absence of other clinical and laboratory abnormalities is characteristic, even pathognomonic, for ITP. CTP is hardly ever considered under these circumstances and many, if not all patients in whom the diagnosis of CTP is later established, are initially diagnosed as having ITP. There are several ready explanations for missing CTP diagnosis at this stage: (1) ITP is by far the most likely diagnosis of isolated thrombocytopenia. (2) CTP is rare, and many physicians, even those who are otherwise familiar with hematological disorders, may not even be aware of the existence of CTP. (3) The rapid increase in platelets after initiation of ITP therapy is, erroneously, interpreted as a good response. In truth, the rise in platelets is not in effect to therapy but reflects the natural course of CTP. (4) Bleeding in CTP is usually mild and mainly consists of mucocutaneous bleeding.15,40 The benign bleeding phenotype, which quickly disappears when platelets rise, encourages physicians to expand the intervals between platelet count monitoring, thereby missing the platelet nadir typical for CTP. (5) Severe thrombocytopenia lasts only for a few days and is easily overlooked if the platelet count is not determined at short time intervals.
When a patient presents with a hemorrhagic diathesis and isolated thrombocytopenia, acute ITP is the most likely diagnosis. Strategies to diagnose ITP will not be addressed and are described in detail elsewhere.41-45 In adults with a platelet count ≤30 × 109/L who are asymptomatic or have minor mucocutaneous bleeding, the American Society of Hematology, for instance, suggests treatment with corticosteroids. IVIG should be used together with corticosteroids if a more rapid increase in platelet count is required.42 Patients who do not respond to corticosteroids or are corticosteroid dependent should receive second-line therapy consisting of a TPO-RA (romiplostim, eltrombopag, avatrombopag), rituximab, or splenectomy.42-44 Because of their rapid action, high efficacy, and favorable safety profile, many experts favor TPO-RAs, which normalize the platelet count in a large number of patients within 7 to 14 days.46
In CTP patients, first- and second-line ITP treatment is at first seemingly effective as the platelet count quickly goes up in all of them. Invariably, platelets will soon drop, often to low levels, thereby mimicking treatment failure. At this stage, we propose that CTP should already be considered as a differential diagnosis to ITP. The rationale behind this advice is that an early diagnosis of CTP will prevent these patients from being exposed to potentially harmful therapies including sustained treatment with a TPA-RA resulting in rebound thrombocytosis and possibly thrombosis,15 rituximab, splenectomy, or even some third-line treatment strategies without the prospect of effect.
Table 2 summarizes important clinical and laboratory features that should raise suspicion of CTP. CTP patients do not respond to ITP treatment, and unusual blood count fluctuations occur during both first- and second-line therapy. During treatment with TPO-RAs, severe thrombocytopenia may be followed by excessively high platelet counts. One has to exclude, however, that fluctuations of platelet counts are induced by abrupt dose changes of TPO-RAs. The bleeding phenotype of CTP is mild, even when thrombocytopenia is severe. In some CTP patients, counts of white cells and reticulocytes also fluctuate.22 TPO levels inversely fluctuate with low platelet counts. Bone marrow biopsies may reveal periodic megakaryocytic hypoplasia or aplasia. Most CTP patients are female and platelets fluctuate in synchrony with the menstrual cycle. A T-cell receptor rearrangement that may be transient is sometimes detectable. CTP can be associated with disorders of the thyroid gland and hematological malignancies, particularly in PCV patients treated with hydroxyurea.
Clinical and laboratory features for considering the diagnosis of CTP
| Features to consider when diagnosing CTP . |
|---|
| Inexplicable platelet count in relation to treatment |
| Thrombocytosis during TPO-RA treatment |
| Mild or no bleeding despite severe thrombocytopenia |
| Fluctuations of white cells and reticulocytes |
| Inverse fluctuation of TPO and platelet counts |
| Periodic megakaryocytic hypoplasia or aplasia |
| Fluctuations in synchrony with menstrual cycle |
| T-cell receptor rearrangement |
| Blood diseases or disorders of the thyroid gland |
| Features to consider when diagnosing CTP . |
|---|
| Inexplicable platelet count in relation to treatment |
| Thrombocytosis during TPO-RA treatment |
| Mild or no bleeding despite severe thrombocytopenia |
| Fluctuations of white cells and reticulocytes |
| Inverse fluctuation of TPO and platelet counts |
| Periodic megakaryocytic hypoplasia or aplasia |
| Fluctuations in synchrony with menstrual cycle |
| T-cell receptor rearrangement |
| Blood diseases or disorders of the thyroid gland |
Figure 1 depicts an algorithm that may assist in distinguishing between ITP and CTP. After failure of corticosteroids and TPO-RAs, and considering clinical and laboratory features listed in Table 2, we propose that treatment should be suspended provided that the bleeding phenotype is mild. We recommend monitoring the blood count at regular intervals, at least weekly, preferably twice weekly, over a period of several weeks and watching out for blood count fluctuations. In CTP patients, a pattern will emerge with higher and lower platelet counts at regular intervals. We believe that delaying other second-line therapies such as rituximab and splenectomy for a short time is justified, as the patients already did not respond to first-line, nor to an otherwise highly effective second-line, therapy. The response to rituximab is less common and delayed; splenectomy is invasive and should be considered later in the course of disease.
Patient 1 (continued)
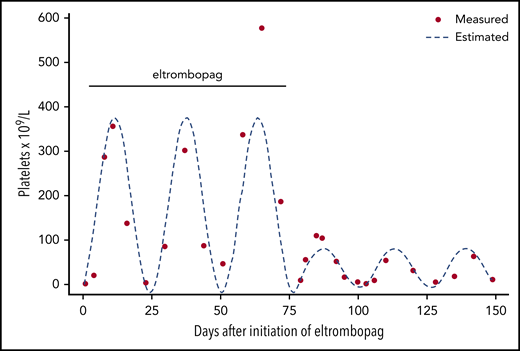
Eltrombopag treatment was discontinued and the platelet count was measured at short intervals over several weeks. As shown in Figure 2, a periodic fluctuation of the platelet count was found with a nadir of <5 × 109/L and a zenith of ∼60 × 109/L every 25 days. At times of severe thrombocytopenia, the patient complained of mild mucocutaneous bleeding consisting of hematomas and occasional nose and gum bleeding. Accordingly, the diagnosis of CTP was established.
Platelet count monitoring in a patient with CTP during and after eltrombopag. Dots represent actual platelet count measurements; the broken line indicates expected platelet count cycling estimated by fitting sine wave to the data. During eltrombopag therapy, the platelet count seemingly increased, but nevertheless, severe thrombocytopenia was seen at 1 occasion 3 weeks after eltrombopag initiation. After discontinuation of eltrombopag, thrombocytosis subsided, and platelet count cycling with a nadir and zenith approximately every 25 days, respectively, became obvious.
Platelet count monitoring in a patient with CTP during and after eltrombopag. Dots represent actual platelet count measurements; the broken line indicates expected platelet count cycling estimated by fitting sine wave to the data. During eltrombopag therapy, the platelet count seemingly increased, but nevertheless, severe thrombocytopenia was seen at 1 occasion 3 weeks after eltrombopag initiation. After discontinuation of eltrombopag, thrombocytosis subsided, and platelet count cycling with a nadir and zenith approximately every 25 days, respectively, became obvious.
Therapy
Patient 2
Patient 2 is a 53-year-old woman with a history of chronic autoimmune thyroiditis and hypertension. She was referred to a hematology clinic because of minor mucocutaneous bleeding and a platelet count of 11 × 109/L. After an extensive diagnostic workup including a bone marrow biopsy, the diagnosis of ITP was made. Over the next 2.5 years, she was treated with several ITP-specific therapies without achieving a response (Table 3). Low platelet counts were often followed by thrombocytosis, especially during TPO-RA therapy. Eventually, she was classified as having refractory ITP.
Response to various ITP-specific therapies of a patient with CTP (patient 2)
| . | Duration . | Platelet count, ×109/L . | |
|---|---|---|---|
| Nadir . | Zenith . | ||
| Prednisolone | 3 mo | 11 | 140 |
| Rituximab | 4 cycles | 3 | 7 |
| SE | NA | 167 (before SE) | 445 (after SE) |
| Eltrombopag | 2 mo | 4 | 900 |
| Romiplostim | 22 mo | 1 | 2.065 |
| . | Duration . | Platelet count, ×109/L . | |
|---|---|---|---|
| Nadir . | Zenith . | ||
| Prednisolone | 3 mo | 11 | 140 |
| Rituximab | 4 cycles | 3 | 7 |
| SE | NA | 167 (before SE) | 445 (after SE) |
| Eltrombopag | 2 mo | 4 | 900 |
| Romiplostim | 22 mo | 1 | 2.065 |
NA, not applicable; SE, splenectomy.
Comment
There is compelling evidence that corticosteroids are ineffective in CTP. All CTP patients treated at our institution failed to respond to prednisolone.15 Likewise, none of the 26 CTP patients reviewed by Go responded to corticosteroids.40 To what extent cyclic platelet fluctuation can be prevented by second-line ITP treatment strategies is unknown as data are lacking. Five of our 9 patients underwent unsuccessful splenectomy. This observation complements the report of Go in which only 1 of 19 patients had a longer-lasting response to splenectomy.40 Regarding TPO-RAs, 4 of our patients received eltrombopag and/or romiplostim over a period of at least 3 months and none of them had a response.15 Bose and coworkers reported 2 female CTP patients in whom romiplostim did not prevent platelet cycling.28 During TPO-RA therapy, all of our patients had rebound thrombocytosis with platelet counts as high as 2000 × 109/L. One of them experienced pulmonary embolism at a time when her platelet count was 437 × 109/L. Rituximab was ineffective in 2 of our patients and in patients reported by others.14,15 In sum, first- and second-line ITP therapies perform poorly in CTP patients.
As in most of our CTP patients, bleeding is usually mild. Nevertheless, major, even life-threatening bleeding can occur. In such an emergency, administration of platelet concentrates should be considered. There is, however, no evidence that platelet transfusions will have a meaningful effect on bleeding or will increase the platelet count. In 1 of our CTP patients, we administered a platelet concentrate at a time when the platelet count was 4 × 109/L and the patient had gum bleeding. We found neither an immediate increase in the platelet count nor a clinical response.
Patient 2 (continued)
Several features, including failure of ITP therapies, the mild bleeding phenotype despite severe thrombocytopenia, unusual platelet count fluctuations, and excessive rebound thrombocytosis, led us to suspect CTP as the underlying disorder. The patient was started on cyclosporine A (CSA) with a target trough level of 100 ng/mL. Platelets gradually stopped cycling and increased over a period of several months. CSA was well tolerated and discontinued several years later. The platelet count has remained normal ever since.
Comment
CSA, an immunosuppressive drug, was shown to be effective in some CTP patients. In 2002, we described a woman with CTP, a clonal T-cell receptor rearrangement, and inversely fluctuating TPO levels who responded to CSA.22 We also found an adequate response to CSA in a patient with a clonal T-cell receptor rearrangement and T-LGL. Zent and coworkers and Telek and coworkers reported 1 patient each with amegakaryocytic CTP who both responded to CSA.12,17 However, CSA did not work in 2 of our CTP patients nor in a patient reported by Rice and coworkers.13 The biological prerequisites that a CTP patient may need to respond to CSA are unknown.
Danazol, an attenuated androgen, was effective in 2 female patients with CTP resulting in remissions lasting >1 and 5 years, respectively.6,20 In a woman with menstrual cycle–associated CTP, danazol lead to normalization of platelets and disappearance of cycling, but she became amenorrheic and relapsed after 1 year despite ongoing treatment.3 In a male patient, a 4-month course of danazol (400 mg per day) was ineffective.5
In sum, both CSA and danazol were given only to a small number of CTP patients and were effective in approximately half of them. Considering the moderate side effects of both drugs, we believe that a sequential treatment attempt is justified. CSA is our preference and we switch to danazol only when CSA is not well tolerated or ineffective after 6 months.
Long-term outcome
Patient 3
Patient 3 is a 37-year-old otherwise healthy male professional athlete who noticed hematomas on arms and legs after karate fights. He had isolated thrombocytopenia (35 × 109/L) and antibodies against glycoprotein IIb/IIIa and glycoprotein Ib/IX. Consequently, the diagnosis of ITP was made. He reduced his sport activities and bleeding ceased. The platelet count was regularly checked and ranged between 13 × 109/L and 58 × 109/L. Five months later, hematomas reappeared on his arms and legs without trauma. The platelet count was 1 × 109/L. Upon treatment with prednisolone (1 mg/kg body weight per day; dose reduction to 50 mg, 25, and 12.5 mg per day every 7 days) and IVIG (0.4 g/kg of body weight on 5 consecutive days), platelets increased to 119 × 109/L but rapidly dropped to 11 × 109/L. Consequently, the patient underwent splenectomy. Postoperatively, platelets ranged between 31 × 109/L and 177 × 109/L. Five months after surgery, mucocutaneous bleeding started again and the platelet count was 2 × 109/L. Upon rituximab (375 mg once weekly), the platelet count quickly increased to 298 × 109/L but dropped to 1 × 109/L prior to the fourth cycle. The patient commenced self-treatment with complementary medicinal products. His bleeding tendency consisted of occasional minor mucocutaneous bleeding only. Weekly monitoring revealed platelet cycling with a nadir every 28 days. Following the diagnosis of CTP, the patient was treated with CSA over 6 months without effect and later refused any further therapies. He regularly checked his blood count and resumed his sport activities when platelets were above 50 × 109/L. Six years after CTP diagnosis, platelet cycling spontaneously subsided and the platelet counts normalized.
Comment
Data on the long-term course of CTP are scarce. According to Go, therapy fails in most patients and platelet cycling continues. Interestingly, remissions can occur.40 Two of our patients, for example, had a spontaneous remission 6 and 8 years after CTP diagnosis, respectively.15 Over the years, the bleeding phenotype of CTP patients is mild and consists, if at all, of minor mucocutaneous bleeding allowing for an almost normal lifestyle.15,40 Regarding invasive procedures, elective surgery or tooth extractions can be safely done at a time when the platelet count is high. Nevertheless, major bleeding has been reported in a few patients: 1 woman had life-threatening gynecological bleeding and 2 patients died of hemorrhagic stroke.5,16,47 One of our patients had nonfatal intracranial bleeding at a time when her platelet count was 1 × 109/L. She was treated with platelet concentrates until platelets spontaneously increased. An association between CTP and hematological malignancies has been reported (Table 1). Accordingly, 1 of our patients developed T-LGL and 1 died of acute myeloid leukemia 18 years after CTP diagnosis.
Conclusions
CTP is a rare disease of uncertain, most likely heterogeneous origin. Patients usually have mild bleeding at mucocutaneous sites, but major bleeding complications can occur. CTP is always misdiagnosed as ITP and the interval between ITP and CTP diagnosis, if ever established, is often long. CTP patients are often exposed to ITP therapies to which they invariably fail to respond. The diagnosis of CTP is made by frequent blood count monitoring revealing a typical pattern of periodic platelet cycling. Some patients respond to CSA or danazol, but in the majority of patients, platelet cycling continues. Of note, spontaneous remissions can occur, even after many years.
Authorship
Contribution: P.A.K. and S.E. wrote and edited the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sabine Eichinger, Department of Medicine I, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: sabine.eichinger@meduniwien.ac.at.