Key Points
Our prospective study shows that in aHUS patients, eculizumab discontinuation based on complement genetics is reasonable and safe.
This discontinuation strategy improves the management and quality of life of many aHUS patients while reducing the cost of treatment.
Abstract
The optimal duration of eculizumab treatment in patients with atypical hemolytic uremic syndrome (aHUS) remains poorly defined. We conducted a prospective national multicenter open-label study to assess eculizumab discontinuation in children and adults with aHUS. Fifty-five patients (including 19 children) discontinued eculizumab (mean treatment duration, 16.5 months). Twenty-eight patients (51%) had rare variants in complement genes, mostly in MCP (n = 12; 22%), CFH (n = 6; 11%), and CFI (n = 6; 10%). At eculizumab discontinuation, 17 (30%) and 4 patients (7%) had stage 3 and 4 chronic kidney disease, respectively. During follow-up, 13 patients (23%; 6 children and 7 adults) experienced aHUS relapse. In multivariable analysis, female sex and presence of a rare variant in a complement gene were associated with an increased risk of aHUS relapse, whereas requirement for dialysis during a previous episode of acute aHUS was not. In addition, increased sC5b-9 plasma level at eculizumab discontinuation was associated with a higher risk of aHUS relapse in all patients and in the subset of carriers with a complement gene rare variant, both by log-rank test and in multivariable analysis. Of the 13 relapsing patients, all of whom restarted eculizumab, 11 regained their baseline renal function and 2 had a worsening of their preexisting chronic kidney disease, including 1 patient who progressed to end-stage renal disease. A strategy of eculizumab discontinuation in aHUS patients based on complement genetics is reasonable and safe. It improves the management and quality of life of a sizeable proportion of aHUS patients while reducing the cost of treatment. This trial was registered at www.clinicaltrials.gov as #NCT02574403.
Introduction
In recent years, the use of C5 blocker eculizumab in patients with atypical hemolytic uremic syndrome (aHUS) has been among the best illustrations of successful translational research.1,2 The design of this specific efficient treatment of a devastating disease was the culmination of decade-long basic and clinical studies, which established that constitutional and, more rarely, acquired complement alternative pathway dysregulations are major drivers of aHUS.3-7
A vast majority of aHUS patients respond to eculizumab, and anti-C5 treatment8,9 has transformed outcomes in aHUS. The risk of stage 5 chronic kidney disease (CKD) in aHUS patients has decreased from 60% to 70% originally to ∼10% to 15%.1,10 This breakthrough has generated in parallel a number of debated issues, especially regarding the optimal duration of eculizumab treatment. The risk of infection (meningitis) resulting from complement inhibition, the need for repeated infusions, and the high cost of treatment have raised the question of under what circumstances discontinuation may be safe, while concerns about aHUS relapse and further kidney injury have favored continuation. Small retrospective series,11-13 including mostly adults, have suggested that eculizumab discontinuation is feasible, although a significant risk of relapse exists for carriers of rare variants in complement genes. We conducted a prospective national multicenter open-label study to assess eculizumab discontinuation feasibility in children and adults with aHUS.
Materials and methods
This prospective open-label multicenter phase 4 noncontrolled study was conducted between October 2015 and December 2019 in 22 pediatric and adult nephrology centers in France (listed in Appendix).
Inclusion criteria were as follows: 1) children and adults (age ≥18 years) treated with eculizumab for primary aHUS (first episode or relapse); 2) treatment with eculizumab for at least 6 months (3 months in patients with an isolated MCP variant; no minimum duration of treatment was required for patients with anti-FH antibodies); and 3) aHUS remission (at the time of eculizumab discontinuation), defined by normal platelet count, absence of hemolysis, and either estimated glomerular filtration rate (eGFR) of >60 mL per minute per 1.73 m2 using the 2009 Schwartz formula in children and the Modification of Diet in Renal Disease formula in adults plus proteinuria/creatininuria ratio of <0.05 g/mmol or stable kidney function for at least 6 months.
aHUS was defined by at least 2 of the following criteria: thrombocytopenia (platelet count <150 × 109/L), mechanical hemolytical anemia (hemoglobin <10 g/dL, lactate dehydrogenase serum level beyond the upper limit of normal, undetectable haptoglobin, and presence of schistocytes on blood smear), and acute kidney injury (serum creatinine and/or proteinuria/creatininuria ratio beyond the upper limit of normal for age or increase of >15% compared with baseline).
aHUS was diagnosed after the exclusion of all other causes of thrombotic microangiopathy (TMA), including infection with Shiga toxin–producing bacteria, ADAMTS13 deficiency, and disorder/condition associated with secondary HUS (malignancy, drug use, autoimmune disease, infection, and cobalamin C deficiency).
Exclusion criteria were as follows: 1) stage 5 CKD requiring chronic dialysis, 2) planned or ongoing pregnancy, 3) absence of informed consent, and 4) patient under protection of judicial authority.
The primary end point was the incidence of aHUS relapse during 2 years of follow-up after eculizumab discontinuation. Diagnosis of aHUS relapse was made based on the criteria used for the initial diagnosis of aHUS. Included patients were seen by their treating nephrologist at least monthly for 3 months and subsequently every 3 months. Serum creatinine, lactate dehydrogenase, haptoglobin and hemoglobin levels, platelet count, urinary protein/creatinine ratio, and urine dipstick test were measured every 2 weeks during the first 6 months and subsequently every month. Patients also performed an at-home urine dipstick test (detection of albuminuria and microscopic hematuria/hemoglobinuria) twice per week. Some data were collected retrospectively, especially for relapsing patients.
Before inclusion in the study, all patients underwent, in their routine clinical management, screening for variants and complex rearrangements in complement factor H (CFH), complement factor I (CFI), membrane-cofactor protein (MCP), C3 factor B (CFB), thrombomodulin (THMD), and diacyl glycerol kinase ε (DGKe) genes using next-generation sequencing and multiplex ligation-dependent probe amplification, as previously described.14 Plasma expressions of C3, C4, CH50, factors H and I, sC5b-9, and CD46 were assessed at baseline (<14 days after the last eculizumab infusion) and at 1, 3, 6, 9, 12, 18, and 24 months after inclusion, as previously decribed.14
The research protocol was approved by the Comité de Protection des Personnes, Ouest II (Angers, France) and by the Agence Nationale de la Sécurité du Médicaments et des Produits de Santé. All participants gave written informed consent. F.F. and V.F.-B. analyzed the data with the assistance of the Direction de la Recherche at Centre Hospitalier Universitaire de Nantes.
Qualitative characteristics are described as numbers and percentages and were compared using the χ2 or Fisher’s exact test as appropriate. Quantitative variables are expressed as means with ranges and were compared using the Student t or Wilcoxon Mann-Whitney test as appropriate. Factors associated with aHUS relapse were analyzed in logistic regression. Factors associated with aHUS relapse at P < .15 were considered for multivariable analysis. Probability of survival without aHUS relapse was estimated using the Kaplan-Meier method and was compared using log-rank analysis. Statistical analyses were performed with SAS software (version 9.4; SAS Institute), and figures were drawn using GraphPad Prism (version 8).
Results
Fifty-seven patients (19 children and 38 adults) were recruited (30 patients were recruited among 52 patients with a new diagnosis of aHUS during the inclusion period). One child was included twice after 2 distinct periods of treatment with eculizumab. Data during follow-up were not available for 2 adult patients who were excluded from the analysis. The main characteristics of the 55 remaining patients are listed in Table 1 (individual details are shown in supplemental Figure 1 (available on the Blood Web site)). Nine patients (16%) had experienced >1 aHUS episode before their inclusion in the study. Twenty-four patients (43%) had required dialysis during their last episode of aHUS (before eculizumab use and subsequent discontinuation), and 24 (43%) presented with extrarenal manifestations, mainly neurological (n = 11; 20%) and cardiac (n = 10; 18.5%). Three kidney transplant recipients (stage 5 CKD resulting from aHUS) were included (supplemental Figure 1). Two were carriers of variants in the CFI gene and received prophylactic eculizumab for 6 and 7 months, respectively. The remaining patient had no detected variant and received prophylactic eculizumab for only 4 months after kidney transplantation, before inclusion in the study.
Characteristics of 19 children and 36 adult patients with aHUS who discontinued eculizumab and were included in study
| . | n (%) . | ||
|---|---|---|---|
| Age <18 y (n = 19)* . | Age ≥18 y (n = 36) . | All (n = 55) . | |
| Sex | |||
| Female | 7 (37) | 17 (47) | 24 (44) |
| Male | 12 (63) | 19 (53) | 31 (56) |
| Complement gene variants | 8 (42) | 20 (55) | 28 (51) |
| CFH | 1 (5) | 5 (14) | 6 (11) |
| MCP | 5 (26)† | 7 (19) | 12 (22) |
| CFI | 0 (0) | 6 (17) | 6 (11) |
| C3 | 0 (0) | 2 (6) | 2 (5) |
| Combined | 2 (11) | 0 (3) | 2 (4) |
| No variant/positive anti–factor H antibodies | 4 (21) | 0 (0) | 4 (7) |
| No variant /no anti–factor H antibodies | 7 (37) | 16 (44) | 23 (42) |
| >1 aHUS episode before inclusion in study‡ | 4 (21) | 4 (11) | 9 (16) |
| At aHUS onset§ | |||
| Serum creatinine, μmol/L | |||
| Mean | 361‖ | 454¶ | 421 |
| Range | 54-1920 | 91-1660 | 54-1920] |
| Requirement for dialysis | 8 (42)‖ | 16 (44%)‖ | 24 (43%) |
| Extrarenal manifestation | 10 (52) | 14 (40) | 24 (43%)# |
| Neurological manifestation | 4 (21) | 7 (20) | 11 (20) |
| Cardiac manifestation | 6 (31.5) | 4 (11) | 10 (18.5) |
| Other | 6 (31.5)** | 8 (23%)†† | 14 (25) |
| At eculizumab discontinuation (inclusion) | |||
| Duration of eculizumab treatment, mo | |||
| Mean | 13.9 | 17.9 | 16.5 |
| Range | 0.95-57.4 | 4.2-59.3 | 0.95-59 |
| Serum creatinine, μmol/L | |||
| Mean | 50 | 124 | 97 |
| Range | 26-134 | 61-305 | 26-305 |
| eGFR, mL/min/1.73 m2 | |||
| Mean | 112 | 62 | 80 |
| Range | 55-169 | 19-129 | 19-169 |
| eGFR, 30-60 mL/min/1.73 m2 | 1 (5%) | 16 (44) | 17 (30) |
| eGFR, 15-29 mL/min/1.73 m2 | 0 | 4 (11) | 4 (7) |
| Urinary protein/creatinine ratio, g/mmol | |||
| Mean | 0.18 | 0.06# | 0.10 |
| Range | 0-3 | 0-0.38 | 0-3 |
| Plasma C3 level <660 mg/L | 0 | 5/35 (14)‖ | 5/53 (9) |
| sC5b-9 ≥300 ng/mL | 11/18 (61)‖ | 23/35 (66) | 34/54 (63) |
| During follow-up | |||
| Duration of follow-up after eculizumab discontinuation, mo | |||
| Mean | 19.5 | 20 | 19.8 |
| Range | 5.4-24 | 1.6-24 | 5.4-24 |
| Patients with aHUS relapse | 6 (30)‡‡ | 7 (19) | 13 (23) |
| Time between eculizumab discontinuation and aHUS relapse, mo | |||
| Mean | 12.3 | 8.1 | 10.2 |
| Range | 5.4-20.6 | 1.6-22.1 | 1.6-22.1 |
| At last follow-up | |||
| Serum creatinine, μmol/L | |||
| Mean | 52 | 147 | 113 |
| Range | 25-144 | 58-881 | 25-881 |
| eGFR, mL/min/1.73 m2 | |||
| Mean | 123 | 58 | 81 |
| Range | 43-199 | 6-128 | 6-199 |
| eGFR, 30-60 mL/min/1.73 m2 | 1 (5%) | 17 (47%) | 18 (32%) |
| eGFR, 15-29 mL/min/1.73 m2 | 0 | 4 (11%) | 4 (7%) |
| eGFR, <15 mL/min/1.73 m2 | 0 | 1 (3%) | 1 (2%) |
| Urinary protein/creatinine ratio, g/mmol | |||
| Mean | 0.10 | 0.05 | 0.07 |
| Range | 0-1.60 | 0-0.44 | 0-1.60 |
| . | n (%) . | ||
|---|---|---|---|
| Age <18 y (n = 19)* . | Age ≥18 y (n = 36) . | All (n = 55) . | |
| Sex | |||
| Female | 7 (37) | 17 (47) | 24 (44) |
| Male | 12 (63) | 19 (53) | 31 (56) |
| Complement gene variants | 8 (42) | 20 (55) | 28 (51) |
| CFH | 1 (5) | 5 (14) | 6 (11) |
| MCP | 5 (26)† | 7 (19) | 12 (22) |
| CFI | 0 (0) | 6 (17) | 6 (11) |
| C3 | 0 (0) | 2 (6) | 2 (5) |
| Combined | 2 (11) | 0 (3) | 2 (4) |
| No variant/positive anti–factor H antibodies | 4 (21) | 0 (0) | 4 (7) |
| No variant /no anti–factor H antibodies | 7 (37) | 16 (44) | 23 (42) |
| >1 aHUS episode before inclusion in study‡ | 4 (21) | 4 (11) | 9 (16) |
| At aHUS onset§ | |||
| Serum creatinine, μmol/L | |||
| Mean | 361‖ | 454¶ | 421 |
| Range | 54-1920 | 91-1660 | 54-1920] |
| Requirement for dialysis | 8 (42)‖ | 16 (44%)‖ | 24 (43%) |
| Extrarenal manifestation | 10 (52) | 14 (40) | 24 (43%)# |
| Neurological manifestation | 4 (21) | 7 (20) | 11 (20) |
| Cardiac manifestation | 6 (31.5) | 4 (11) | 10 (18.5) |
| Other | 6 (31.5)** | 8 (23%)†† | 14 (25) |
| At eculizumab discontinuation (inclusion) | |||
| Duration of eculizumab treatment, mo | |||
| Mean | 13.9 | 17.9 | 16.5 |
| Range | 0.95-57.4 | 4.2-59.3 | 0.95-59 |
| Serum creatinine, μmol/L | |||
| Mean | 50 | 124 | 97 |
| Range | 26-134 | 61-305 | 26-305 |
| eGFR, mL/min/1.73 m2 | |||
| Mean | 112 | 62 | 80 |
| Range | 55-169 | 19-129 | 19-169 |
| eGFR, 30-60 mL/min/1.73 m2 | 1 (5%) | 16 (44) | 17 (30) |
| eGFR, 15-29 mL/min/1.73 m2 | 0 | 4 (11) | 4 (7) |
| Urinary protein/creatinine ratio, g/mmol | |||
| Mean | 0.18 | 0.06# | 0.10 |
| Range | 0-3 | 0-0.38 | 0-3 |
| Plasma C3 level <660 mg/L | 0 | 5/35 (14)‖ | 5/53 (9) |
| sC5b-9 ≥300 ng/mL | 11/18 (61)‖ | 23/35 (66) | 34/54 (63) |
| During follow-up | |||
| Duration of follow-up after eculizumab discontinuation, mo | |||
| Mean | 19.5 | 20 | 19.8 |
| Range | 5.4-24 | 1.6-24 | 5.4-24 |
| Patients with aHUS relapse | 6 (30)‡‡ | 7 (19) | 13 (23) |
| Time between eculizumab discontinuation and aHUS relapse, mo | |||
| Mean | 12.3 | 8.1 | 10.2 |
| Range | 5.4-20.6 | 1.6-22.1 | 1.6-22.1 |
| At last follow-up | |||
| Serum creatinine, μmol/L | |||
| Mean | 52 | 147 | 113 |
| Range | 25-144 | 58-881 | 25-881 |
| eGFR, mL/min/1.73 m2 | |||
| Mean | 123 | 58 | 81 |
| Range | 43-199 | 6-128 | 6-199 |
| eGFR, 30-60 mL/min/1.73 m2 | 1 (5%) | 17 (47%) | 18 (32%) |
| eGFR, 15-29 mL/min/1.73 m2 | 0 | 4 (11%) | 4 (7%) |
| eGFR, <15 mL/min/1.73 m2 | 0 | 1 (3%) | 1 (2%) |
| Urinary protein/creatinine ratio, g/mmol | |||
| Mean | 0.10 | 0.05 | 0.07 |
| Range | 0-1.60 | 0-0.44 | 0-1.60 |
Normal values: urinary protein/creatinine ratio, <0.05 g/mmol; plasma C3, 660-1250 mg/L; sC5b-9, <300 ng/mL.
One child was included twice in the study during 2 distinct periods of eculizumab treatment.
Two patients (including child with familial aHUS) had persistently low CD46 expression on granulocytes without genetic molecular mechanism identified.
aHUS episode outside a period of eculizmab treatment.
At onset of the last aHUS episode before eculizumab discontinuation.
Data missing for 1 patient.
Data missing for 3 patients.
Data missing for 2 patients.
Six patients had 7 extrarenal manifestations: digestive (n = 4), cutaneous (n = 2), and vascular (n = 1).
Eight patients had 13 extrarenal manifestations: digestive (n = 6), cutaneous (n = 3), vascular (n = 2), hepatic (n = 1), and hematological (n = 1).
Child included twice experienced 2 aHUS relapses.
Among the 55 patients, 28 (51%) had a rare variant in at least 1 complement gene: MCP (n = 12; 22%), CFH (n = 6; 11%) CFI (n = 6; 10%), C3 gene (n = 2; 4%), and combined variants (n = 2; 4%; C3/CFI, n = 1 and MCP/CFI, n = 1). The clinical characteristics of patients with or without detected rare complement gene variants are listed in supplemental Table 1. Among the 26 identified variants, 22 were classified as pathogenic and 4 as of undetermined significance (supplemental Table 2). In 4 additional patients, anti-CFH antibodies had been present at aHUS diagnosis at high titer (9000-60 000 arbitrary units), but at inclusion, antibody titer had decreased to 473 to 1500 arbitrary units after immunosuppressive treatments (characteristics summarized in supplemental Table 3).
Mean duration of eculizumab treatment was 16.5 months (range, 0.95-59 months). One child with anti-CFH antibodies was included after 1 month of eculizumab treatment. At eculizumab discontinuation, 17 patients (30%; including 1 child) had stage 3 CKD and 4 (7%) had stage 4 CKD. Five patients (9%; all adults) had decreased plasma C3 levels, and 32 (58%) had elevated sC5b-9 levels.
During follow-up, 13 patients (23%; 6 [31%] of 19 children and 7 [19%] of 36 adults) had aHUS relapse (Tables 1 and 2). The risk of relapse did not differ between children and adults (Figure 1). The total number of relapses was 14 (1 child, included twice, experienced 2 relapses); 11 relapses (79%) were precipitated by infection (mostly viral), and 1 occurred in the setting of acute pancreatitis resulting from alcohol abuse (Table 2). During all relapses (except 1), acute kidney injury occurred (mean serum creatinine, 221 μmol/L; range, 45-802 μmol/L). One patient (patient 12; Table 2) required dialysis in the setting of severe acute pancreatitis. Thrombocytopenia was present during 12 relapses (85%).
Individual characteristics of 6 children and 7 adults who experienced aHUS relapse after eculizumab discontinuation
| Patient . | Sex, age, y . | Complement gene variant . | At eculizumab discontinuation . | Precipitating factor of aHUS relapse . | At aHUS relapse . | At 3 mo after aHUS relapse and eculizumab restart . | At last follow-up . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCr, μmol/L (eGFR, mL/min/1.73 m2) . | UP/Cr, g/mmol . | Time from eculizumab discontinuation, mo . | SCr, μmol/L . | Plt × 109/L . | UP/Cr, g/mmol . | SCr, μmol/L (eGFR, mL/min/1.73 m2) . | UP/Cr, g/mmol . | Duration, mo . | SCr, μmol/L (eGFR, mL/min/1.73 m2) . | UP/Cr, g/mmol . | ||||
| 1 | M, 4 | CFI (p.Gly261Asp)/C3 (p.Thr1383Asn) | 34.0 (104) | 0.018 | Bacterial infection | 5.4 | 62 | 95 | 0.04 | 34.0 (107) | 0.01 | 18.0 | 46.0 (85) | 0.012 |
| 2* | F, 6. | CFH (p.Ser1191Trp) | 32.0 (128) | 0.03 | Flu-like illness | 11.5 | 92 | 63 | 1.28 | 33.0 (130) | 0.04 | 3.2 | 33.0 (130) | 0.04 |
| F, 7 | 34.0 (127) | 0.03 | Flu-like illness | 8.1 | 69 | 126 | 1.06 | 36.0 (127) | 0.03 | 18.3 | 38.0 (126) | 0.08 | ||
| 3† | F, 7 | MCP (persistently low CD46 level) | 52.0 (79) | 0.008 | Flu-like illness | 9.9 | 105 | 94 | 3.08 | 50 (78)‡ | 0.01 | 14.7 | 53.0 (85) | 0.009 |
| 4 | F, 8 | MCP (p.Asp33His)/MCP (p.Asp33His) | 34.0 (143) | 0.01 | Gastroenteritis | 20.5 | 188 | 56 | 5.66 | 32.0 (145) | 0.02 | 2.8 | 32.0 (145) | 0.02 |
| 5 | M, 9 | MCP (IVS2+2)/MCP (IVS2+2) | 39.1 (169) | 3.0 | Gastroenteritis | 13.4 | 214 | 72 | 1.21 | 32.8 (210) | 0.05 | 8.7 | 35.6 (199) | 0.05 |
| 6§ | M, 9 | None | 45.0 (109) | 0.01 | Tonsillitis | 17.2 | 45 | 62 | NA | 46 (108)‖ | NA | 15.0 | 47.0 (148) | 0.01 |
| 7 | F, 30 | C3 (p.Ala1094Ser) | 136.0 (42) | 0.08 | Sinusitis | 2.5 | 191 | 138 | 0.28 | 131.0 (44) | 0.15 | 18.5 | 148.5 (37) | 0.1 |
| 8 | F, 34 | CFH (p.Phe1199Leu) | 121.0 (47) | 0.03 | Tracheitis | 20.0 | 165 | 209 | 0.06 | 125.0 (45) | 0.03 | 6.6 | 129.0 (43) | 0.06 |
| 9 | F, 34 | MCP (p.Tyr117Stop) | 93.0 (63) | 0.13 | Diarrhea | 1.6 | 184 | 57 | 0.36 | 89.3 (66) | 0.1 | 23.7 | 77.8 (77) | 0.05 |
| 10 | F, 38 | MCP (IVS2+2)/MCP (IVS2+2) | 121.0 (46) | 0.22 | Viral tonsillitis | 2.5 | 163 | 113 | 0.37 | 145.0 (37) | 0.05 | 20.9 | 132.0 (41) | 0.08 |
| 11 | M, 44 | MCP (IVS2+2) | 245.0 (27) | 0.15 | — | 3.6 | 414 | 143 | 0.26 | 426.0 (14) | 0.16 | 10.8 | 881.0 (6)¶ | 0.21 |
| 12 | F, 53# | CFI (p.Pro50Ala) | 64.0 (89) | 0.01 | Pancreatitis | 3.7 | 802 | 30 | 0.25 | 69.0 (82) | NA | 21.1 | 64.0 (89) | 0.04 |
| 13 | F, 56 | CFH (p.Arg1215Stop) | 101 (52) | 0.02 | — | 22.1 | 232 | 235 | 0.26 | 168.0 (29) | 0.07 | 10.8 | 167.0 (29) | 0.04 |
| Patient . | Sex, age, y . | Complement gene variant . | At eculizumab discontinuation . | Precipitating factor of aHUS relapse . | At aHUS relapse . | At 3 mo after aHUS relapse and eculizumab restart . | At last follow-up . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCr, μmol/L (eGFR, mL/min/1.73 m2) . | UP/Cr, g/mmol . | Time from eculizumab discontinuation, mo . | SCr, μmol/L . | Plt × 109/L . | UP/Cr, g/mmol . | SCr, μmol/L (eGFR, mL/min/1.73 m2) . | UP/Cr, g/mmol . | Duration, mo . | SCr, μmol/L (eGFR, mL/min/1.73 m2) . | UP/Cr, g/mmol . | ||||
| 1 | M, 4 | CFI (p.Gly261Asp)/C3 (p.Thr1383Asn) | 34.0 (104) | 0.018 | Bacterial infection | 5.4 | 62 | 95 | 0.04 | 34.0 (107) | 0.01 | 18.0 | 46.0 (85) | 0.012 |
| 2* | F, 6. | CFH (p.Ser1191Trp) | 32.0 (128) | 0.03 | Flu-like illness | 11.5 | 92 | 63 | 1.28 | 33.0 (130) | 0.04 | 3.2 | 33.0 (130) | 0.04 |
| F, 7 | 34.0 (127) | 0.03 | Flu-like illness | 8.1 | 69 | 126 | 1.06 | 36.0 (127) | 0.03 | 18.3 | 38.0 (126) | 0.08 | ||
| 3† | F, 7 | MCP (persistently low CD46 level) | 52.0 (79) | 0.008 | Flu-like illness | 9.9 | 105 | 94 | 3.08 | 50 (78)‡ | 0.01 | 14.7 | 53.0 (85) | 0.009 |
| 4 | F, 8 | MCP (p.Asp33His)/MCP (p.Asp33His) | 34.0 (143) | 0.01 | Gastroenteritis | 20.5 | 188 | 56 | 5.66 | 32.0 (145) | 0.02 | 2.8 | 32.0 (145) | 0.02 |
| 5 | M, 9 | MCP (IVS2+2)/MCP (IVS2+2) | 39.1 (169) | 3.0 | Gastroenteritis | 13.4 | 214 | 72 | 1.21 | 32.8 (210) | 0.05 | 8.7 | 35.6 (199) | 0.05 |
| 6§ | M, 9 | None | 45.0 (109) | 0.01 | Tonsillitis | 17.2 | 45 | 62 | NA | 46 (108)‖ | NA | 15.0 | 47.0 (148) | 0.01 |
| 7 | F, 30 | C3 (p.Ala1094Ser) | 136.0 (42) | 0.08 | Sinusitis | 2.5 | 191 | 138 | 0.28 | 131.0 (44) | 0.15 | 18.5 | 148.5 (37) | 0.1 |
| 8 | F, 34 | CFH (p.Phe1199Leu) | 121.0 (47) | 0.03 | Tracheitis | 20.0 | 165 | 209 | 0.06 | 125.0 (45) | 0.03 | 6.6 | 129.0 (43) | 0.06 |
| 9 | F, 34 | MCP (p.Tyr117Stop) | 93.0 (63) | 0.13 | Diarrhea | 1.6 | 184 | 57 | 0.36 | 89.3 (66) | 0.1 | 23.7 | 77.8 (77) | 0.05 |
| 10 | F, 38 | MCP (IVS2+2)/MCP (IVS2+2) | 121.0 (46) | 0.22 | Viral tonsillitis | 2.5 | 163 | 113 | 0.37 | 145.0 (37) | 0.05 | 20.9 | 132.0 (41) | 0.08 |
| 11 | M, 44 | MCP (IVS2+2) | 245.0 (27) | 0.15 | — | 3.6 | 414 | 143 | 0.26 | 426.0 (14) | 0.16 | 10.8 | 881.0 (6)¶ | 0.21 |
| 12 | F, 53# | CFI (p.Pro50Ala) | 64.0 (89) | 0.01 | Pancreatitis | 3.7 | 802 | 30 | 0.25 | 69.0 (82) | NA | 21.1 | 64.0 (89) | 0.04 |
| 13 | F, 56 | CFH (p.Arg1215Stop) | 101 (52) | 0.02 | — | 22.1 | 232 | 235 | 0.26 | 168.0 (29) | 0.07 | 10.8 | 167.0 (29) | 0.04 |
eGFR determined using the Modification of Diet in Renal Disease formula in adults and the Schwartz formula in children.
NA, not available; Plt, platelet; SCr, serum creatinine; UP/Cr, urinary protein/creatinine ratio.
*Patient was included twice in the study; †Patient had a familial form of aHUS; ‡Data at <1 mo after relapse; §Patient was diagnosed with hereditary ADAMTS13 deficiency after completion of the study; ‖Data at 6 mo after relapse; ¶Patient reached end-stage renal disease and subsequently underwent kidney transplantation; #Kidney transplantation patient.
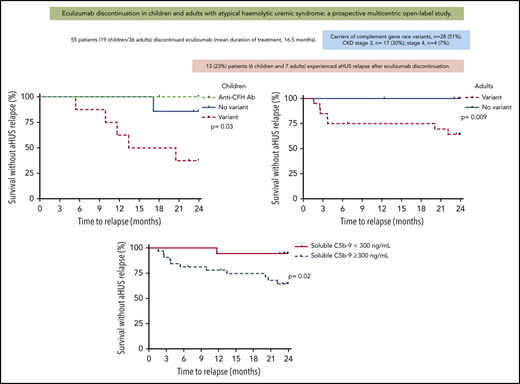
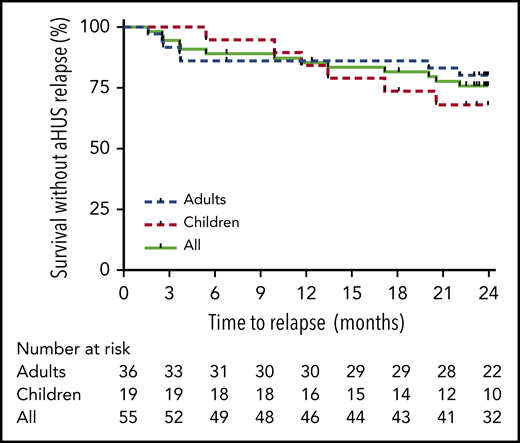
Probability of survival without aHUS relapse after eculizumab discontinuation according to age at eculizumab discontinuation. Risk of relapse was not statistically significant different between children and adults (P = .39 by log-rank test).
Probability of survival without aHUS relapse after eculizumab discontinuation according to age at eculizumab discontinuation. Risk of relapse was not statistically significant different between children and adults (P = .39 by log-rank test).
Characteristics of relapsing and nonrelapsing patients after eculizumab discontinuation are listed in Table 3 and supplemental Table 4. In univariate analysis, female sex (69% vs 36%; P = .03), history of >1 previous aHUS episode (38% vs 7%; P = .01), detection of a complement gene variant (92% vs 25%; P = .0009), and increased sC5b-9 at inclusion (92% vs 55%; P = .02) were more frequent in relapsing than in nonrelapsing patients. In contrast, requirement for dialysis during the last aHUS episode was more frequent in nonrelapsing compared with relapsing patients (52% vs 15%; P = .02). At last follow-up, urinary protein/creatinine ratio was higher in relapsing compared with nonrelapsing adults (0.12 vs 0.04 g/mmol; P = .004).
Characteristics at inclusion and during follow-up of 13 patients who relapsed and 42 patients who did not relapse after eculizumab discontinuation
| . | n (%) . | P . | |
|---|---|---|---|
| Relapsing patients (n = 13)* . | Nonrelapsing patients (n = 42) . | ||
| Age, <18 y | 6 (46) | 13 (31) | .34 |
| Sex | .03 | ||
| Female | 9 (69) | 15 (36) | |
| Male | 4 (31) | 27 (64) | |
| >1 aHUS episode before inclusion in study† | 5 (38) | 3 (7) | .01 |
| Requirement for dialysis during last aHUS episode before eculizumab discontinuation | 2 (15) | 22 (52) | .02 |
| Extrarenal manifestation | 3 (23) | 21 (53)‡ | .06 |
| Duration of eculizumab treatment, mo | .28 | ||
| Mean | 23.3 | 14 | |
| Range | 3.3-59.3 | 0.95-57.4 | |
| Complement gene variants | 12 (92) | 16 (25) | .0009 |
| CFH | 3 (23) | 3 (7) | .1 |
| MCP | 6 (46) | 6 (14) | .02 |
| CFI | 1 (8) | 4 (9) | 1 |
| C3 | 1 (8) | 2 (5) | .5 |
| Combined | 1 (8) | 1 (2) | .4 |
| No variant/positive anti–factor H antibodies | 0 (0) | 4 (9) | .5 |
| No variant/no anti–factor H antibodies | 1 (7)§ | 22 (53) | .004 |
| No. of relapses after eculizumab discontinuation | 14 | — | |
| At eculizumab discontinuation (inclusion) | |||
| Serum creatinine, μmol/L | |||
| All | .12 | ||
| Mean | 82 | 102 | |
| Range | 32-245 | 26-305 | |
| Children | .15 | ||
| Mean | 39 | 56 | |
| Range | 32-52 | 26-134 | |
| Adults | .96 | ||
| Mean | 126 | 123 | |
| Range | 64-245 | 61-305 | |
| eGFR, mL/min/1.73 m2 | |||
| All | .57 | ||
| Mean | 87 | 78 | |
| Range | 27-169 | 19-130 | |
| Children | .19 | ||
| Mean | 123 | 106 | |
| Range | 79-169 | 55-124 | |
| Adults | .25 | ||
| Mean | 52 | 65 | |
| Range | 27-89 | 19-130 | |
| CKD stage 3 | 4 (30) | 13 (31) | .9 |
| CKD stage 4 | 1 (7) | 3 (7) | 1 |
| Urinary protein/creatinine ratio, g/mmol | |||
| All | .10 | ||
| Mean | 0.27 | 0.04‡ | |
| Range | 0.01-3 | 0-0.38 | |
| Children | .39 | ||
| Mean | 0.45 | 0.03 | |
| Range | 0.01-3.00 | 0.00-0.14 | |
| Adults | .07 | ||
| Mean | 0.10 | 0.05 | |
| Range | 0.01-0.22 | 0.00-0.38 | |
| Plasma C3 level <660 mg/L at inclusion | 2/12 (17) | 3/41 (7) | .31 |
| Plasma factor H level <70% at inclusion | 1/10 (10)‖ | 2/40 (5)‖ | .49 |
| sC5b-9 ≥300 ng/ml at inclusion | 11/12 (92)‖ | 23/41 (56)‖ | .04 |
| sC5b-9 at inclusion | .03 | ||
| Mean | 418 | 325 | |
| Range | 363-499 | 234-428 | |
| At last follow-up | |||
| Duration of follow-up, mo | |||
| Before relapse | |||
| All | <.0001 | ||
| Mean | 9.3 | 23 | |
| Range | 1.6-22.1 | 6.7-24 | |
| Children | <.0001 | ||
| Mean | 10.9 | 23.1 | |
| Range | 5.4-17.2 | 18.1-24 | |
| Adults | <.0001 | ||
| Mean | 8 | 22.9 | |
| Range | 1.6-22.1 | 6.7-24 | |
| After relapse | |||
| All | — | ||
| Mean | 13.2 | ||
| Range | 2.7-23.7 | ||
| Children | — | ||
| Mean | 10.9 | ||
| Range | 2.8-18.3 | ||
| Adults | — | ||
| Mean | 14.9 | ||
| Range | 2.7-23.7 | ||
| Serum creatinine, μmol/L | |||
| All | .21 | ||
| Mean | 135 | 107 | |
| Range | 32-881 | 25-287 | |
| Children | .19 | ||
| Mean | 41 | 59 | |
| Range | 32-53 | 25-144 | |
| Adults | .57 | ||
| Mean | 229 | 128 | |
| Range | 64-881 | 58-287 | |
| eGFR, mL/min/1.73 m2 | |||
| All | .70 | ||
| Mean | 89 | 79 | |
| Range | 6-199 | 21-194 | |
| Children | .48 | ||
| Mean | 131 | 118 | |
| Range | 84-199 | 43-194 | |
| Adults | .17 | ||
| Mean | 46 | 61 | |
| Range | 6-89 | 21-128 | |
| eGFR, <30 mL/min/1.73 m2 | |||
| All | 2 (14) | 3 (7) | .59 |
| Children | 0 | 0 | — |
| Adults | 2 (29) | 3 (10) | .24 |
| Urinary protein/creatinine ratio, g/mmol | |||
| All | .004 | ||
| Mean | 0.07 | 0.07 | |
| Range | 0.01-0.26 | 0.00-1.60 | |
| Children | .15 | ||
| Mean | 0.03 | 0.14 | |
| Range | 0.01-0.09 | 0.00-1.60 | |
| Adults | .004 | ||
| Mean | 0.12 | 0.04 | |
| Range | 0.05-0.26 | 0.00-0.44 | |
| . | n (%) . | P . | |
|---|---|---|---|
| Relapsing patients (n = 13)* . | Nonrelapsing patients (n = 42) . | ||
| Age, <18 y | 6 (46) | 13 (31) | .34 |
| Sex | .03 | ||
| Female | 9 (69) | 15 (36) | |
| Male | 4 (31) | 27 (64) | |
| >1 aHUS episode before inclusion in study† | 5 (38) | 3 (7) | .01 |
| Requirement for dialysis during last aHUS episode before eculizumab discontinuation | 2 (15) | 22 (52) | .02 |
| Extrarenal manifestation | 3 (23) | 21 (53)‡ | .06 |
| Duration of eculizumab treatment, mo | .28 | ||
| Mean | 23.3 | 14 | |
| Range | 3.3-59.3 | 0.95-57.4 | |
| Complement gene variants | 12 (92) | 16 (25) | .0009 |
| CFH | 3 (23) | 3 (7) | .1 |
| MCP | 6 (46) | 6 (14) | .02 |
| CFI | 1 (8) | 4 (9) | 1 |
| C3 | 1 (8) | 2 (5) | .5 |
| Combined | 1 (8) | 1 (2) | .4 |
| No variant/positive anti–factor H antibodies | 0 (0) | 4 (9) | .5 |
| No variant/no anti–factor H antibodies | 1 (7)§ | 22 (53) | .004 |
| No. of relapses after eculizumab discontinuation | 14 | — | |
| At eculizumab discontinuation (inclusion) | |||
| Serum creatinine, μmol/L | |||
| All | .12 | ||
| Mean | 82 | 102 | |
| Range | 32-245 | 26-305 | |
| Children | .15 | ||
| Mean | 39 | 56 | |
| Range | 32-52 | 26-134 | |
| Adults | .96 | ||
| Mean | 126 | 123 | |
| Range | 64-245 | 61-305 | |
| eGFR, mL/min/1.73 m2 | |||
| All | .57 | ||
| Mean | 87 | 78 | |
| Range | 27-169 | 19-130 | |
| Children | .19 | ||
| Mean | 123 | 106 | |
| Range | 79-169 | 55-124 | |
| Adults | .25 | ||
| Mean | 52 | 65 | |
| Range | 27-89 | 19-130 | |
| CKD stage 3 | 4 (30) | 13 (31) | .9 |
| CKD stage 4 | 1 (7) | 3 (7) | 1 |
| Urinary protein/creatinine ratio, g/mmol | |||
| All | .10 | ||
| Mean | 0.27 | 0.04‡ | |
| Range | 0.01-3 | 0-0.38 | |
| Children | .39 | ||
| Mean | 0.45 | 0.03 | |
| Range | 0.01-3.00 | 0.00-0.14 | |
| Adults | .07 | ||
| Mean | 0.10 | 0.05 | |
| Range | 0.01-0.22 | 0.00-0.38 | |
| Plasma C3 level <660 mg/L at inclusion | 2/12 (17) | 3/41 (7) | .31 |
| Plasma factor H level <70% at inclusion | 1/10 (10)‖ | 2/40 (5)‖ | .49 |
| sC5b-9 ≥300 ng/ml at inclusion | 11/12 (92)‖ | 23/41 (56)‖ | .04 |
| sC5b-9 at inclusion | .03 | ||
| Mean | 418 | 325 | |
| Range | 363-499 | 234-428 | |
| At last follow-up | |||
| Duration of follow-up, mo | |||
| Before relapse | |||
| All | <.0001 | ||
| Mean | 9.3 | 23 | |
| Range | 1.6-22.1 | 6.7-24 | |
| Children | <.0001 | ||
| Mean | 10.9 | 23.1 | |
| Range | 5.4-17.2 | 18.1-24 | |
| Adults | <.0001 | ||
| Mean | 8 | 22.9 | |
| Range | 1.6-22.1 | 6.7-24 | |
| After relapse | |||
| All | — | ||
| Mean | 13.2 | ||
| Range | 2.7-23.7 | ||
| Children | — | ||
| Mean | 10.9 | ||
| Range | 2.8-18.3 | ||
| Adults | — | ||
| Mean | 14.9 | ||
| Range | 2.7-23.7 | ||
| Serum creatinine, μmol/L | |||
| All | .21 | ||
| Mean | 135 | 107 | |
| Range | 32-881 | 25-287 | |
| Children | .19 | ||
| Mean | 41 | 59 | |
| Range | 32-53 | 25-144 | |
| Adults | .57 | ||
| Mean | 229 | 128 | |
| Range | 64-881 | 58-287 | |
| eGFR, mL/min/1.73 m2 | |||
| All | .70 | ||
| Mean | 89 | 79 | |
| Range | 6-199 | 21-194 | |
| Children | .48 | ||
| Mean | 131 | 118 | |
| Range | 84-199 | 43-194 | |
| Adults | .17 | ||
| Mean | 46 | 61 | |
| Range | 6-89 | 21-128 | |
| eGFR, <30 mL/min/1.73 m2 | |||
| All | 2 (14) | 3 (7) | .59 |
| Children | 0 | 0 | — |
| Adults | 2 (29) | 3 (10) | .24 |
| Urinary protein/creatinine ratio, g/mmol | |||
| All | .004 | ||
| Mean | 0.07 | 0.07 | |
| Range | 0.01-0.26 | 0.00-1.60 | |
| Children | .15 | ||
| Mean | 0.03 | 0.14 | |
| Range | 0.01-0.09 | 0.00-1.60 | |
| Adults | .004 | ||
| Mean | 0.12 | 0.04 | |
| Range | 0.05-0.26 | 0.00-0.44 | |
One child was included twice in study during 2 distinct periods of eculizumab treatment and experienced 2 relapses.
aHUS episode outside period of eculizmab treatment.
Data missing for 2 patients.
Patient was diagnosed with hereditary ADAMTS13 deficiency after completion of the study.
Data missing for 1 patient.
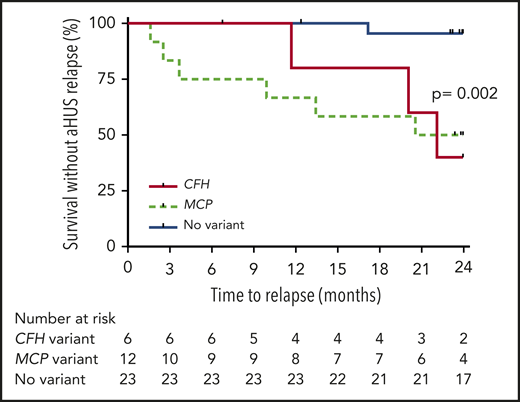
The risk of aHUS relapse after eculizumab discontinuation was higher in patients with complement gene variants than in those without, regardless of age (Figures 2 and 3). The risk of relapse was highest in carriers of variants in CFH (3 [50%] of 6) and MCP genes (6 [50%] of 12) and lowest in patients without detected variants (1 [4%] of 23; supplemental Table 5; Figure 4). All relapsing patients, except 1, had a rare variant detected in a complement gene, mostly MCP (n = 6; Table 2). After completion of the study, an additional genetic workup in the relapsing patient (Table 2) with no detected variant in a complement, THMD, and DGKe gene revealed the presence of 2 pathogenic variants in the ADAMTS13 gene. At age 6 years, this male patient had a first TMA episode preceded by Shiga toxin–negative diarrhea and characterized by mild acute kidney injury (serum creatinine, 54 μmol/L), heavy proteinuria (urinary protein/creatinine ratio, 0.23 g/mmol), profound thrombocytopenia (13 g/L), and acute pancreatitis. TMA spontaneously resolved. He experienced a TMA relapse at age 8 years, in the setting of influenza A infection, with mild acute kidney injury (serum creatinine, 62 μmol/L) and profound thrombocytopenia (12 g/L). aHUS was diagnosed. Eculizumab was started, TMA rapidly resolved (<5 days), and treatment was continued for 25 months. Eighteen months after eculizumab discontinuation (and inclusion in the study), TMA recurred (serum creatinine, 45 μmol/L; proteinuria, 4+ on dipstick; platelet count, 62 × 109/L) and completely resolved 14 days after restart of eculizumab. His ADAMTS13 activity, initially reported as detectable, was subsequently controlled at <5% without detected autoantibodies.
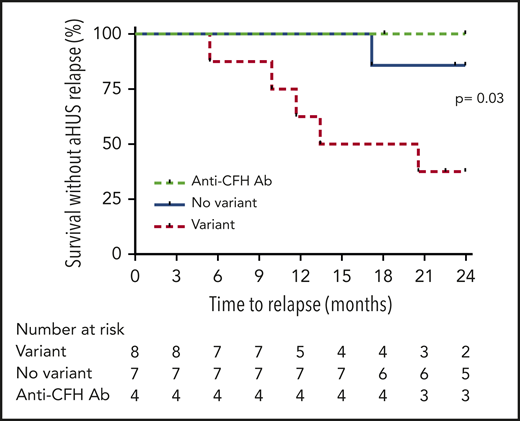
Probability of survival without aHUS relapse after eculizumab discontinuation in children according to presence or absence of detected complement gene variant. Risk of relapse was higher in children with complement gene variants compared with those without variants (P = .03 by log-rank test). Ab, antibody.
Probability of survival without aHUS relapse after eculizumab discontinuation in children according to presence or absence of detected complement gene variant. Risk of relapse was higher in children with complement gene variants compared with those without variants (P = .03 by log-rank test). Ab, antibody.
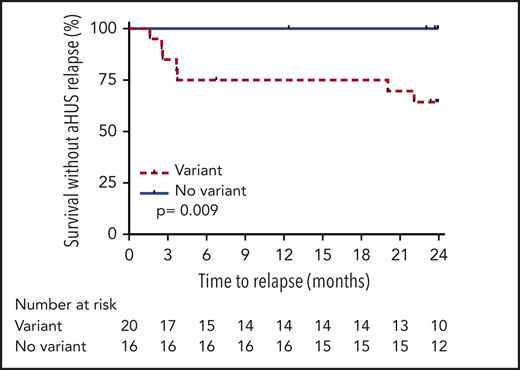
Probability of survival without aHUS relapse after eculizumab discontinuation in adults according to presence or absence of detected complement gene variant. Risk of relapse was higher in adults with complement gene variants compared with those without variants (P = .009 by log-rank test).
Probability of survival without aHUS relapse after eculizumab discontinuation in adults according to presence or absence of detected complement gene variant. Risk of relapse was higher in adults with complement gene variants compared with those without variants (P = .009 by log-rank test).
Probability of survival without aHUS relapse after eculizumab discontinuation in children and adults according to type of detected complement gene variant. Risk of relapse was higher in patients with variants in CFH and MCP genes compared with those without variants (P = .002 by log-rank test).
Probability of survival without aHUS relapse after eculizumab discontinuation in children and adults according to type of detected complement gene variant. Risk of relapse was higher in patients with variants in CFH and MCP genes compared with those without variants (P = .002 by log-rank test).
In the first multivariable analysis, presence of a rare variant in a complement gene (odds ratio [OR], 16.20; 95% confidence interval [CI], 1.78-147.73) was associated with increased risk of aHUS relapse. Female sex (OR, 4.21; 95% CI, 0.85-20.75) tended to be associated with increased risk of aHUS relapse, whereas requirement for dialysis during the last aHUS episode before eculizumab discontinuation (OR, 0.17; 95% CI, 0.03-1.02) tended to be associated with decreased risk, without reaching statistical significance (Table 4). Because of the small sample size, this multivariable analysis could not take into account simultaneously presence of a complement gene variant and increased sC5b-9 level (no patient without a complement gene variant and with available sC5b-9 level had experienced aHUS relapse after eculizumab discontinuation).
Factors associated with aHUS relapse after eculizumab discontinuation in multivariable analysis
| . | OR . | 95% CI . | P . |
|---|---|---|---|
| Analysis including presence of complement gene variant as parameter | |||
| Requirement for dialysis during last aHUS episode before eculizumab discontinuation | 0.17 | 0.03-1.02 | .0560 |
| Female sex | 4.21 | 0.85-20.75 | .0777 |
| Presence of rare variant in complement gene | 16.20 | 1.78-147.73 | .0135 |
| Analysis including sC5b-9 level as parameter | |||
| Requirement for dialysis during last aHUS episode before eculizumab discontinuation | 0.07 | 0.01-0.53 | .0101 |
| Female sex | 10.06 | 1.53-66.19 | .0163 |
| Plasma sC5b-9 ≥300 ng/mL at inclusion | 20.96 | 1.76-250.12 | .0162 |
| . | OR . | 95% CI . | P . |
|---|---|---|---|
| Analysis including presence of complement gene variant as parameter | |||
| Requirement for dialysis during last aHUS episode before eculizumab discontinuation | 0.17 | 0.03-1.02 | .0560 |
| Female sex | 4.21 | 0.85-20.75 | .0777 |
| Presence of rare variant in complement gene | 16.20 | 1.78-147.73 | .0135 |
| Analysis including sC5b-9 level as parameter | |||
| Requirement for dialysis during last aHUS episode before eculizumab discontinuation | 0.07 | 0.01-0.53 | .0101 |
| Female sex | 10.06 | 1.53-66.19 | .0163 |
| Plasma sC5b-9 ≥300 ng/mL at inclusion | 20.96 | 1.76-250.12 | .0162 |
Multivariable analysis could not take into account simultaneously presence of a complement gene variant and increased sC5b-9 level, because of the small sample size (no patient without complement gene variant and with available sC5b-9 level had experienced aHUS relapse after eculizumab discontinuation). Therefore, 2 multivariable analyses were performed. The first included all parameters except for plasma level of sC5b-9 at eculizumab discontinuation. The second included all parameters except for presence of rare variant in complement gene.
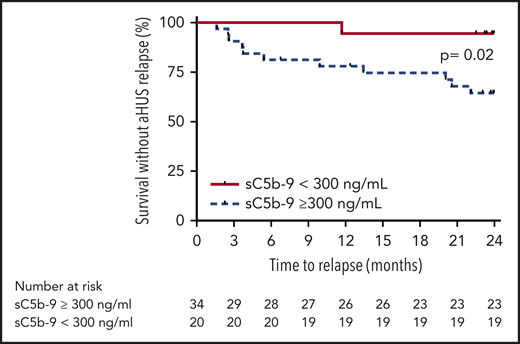
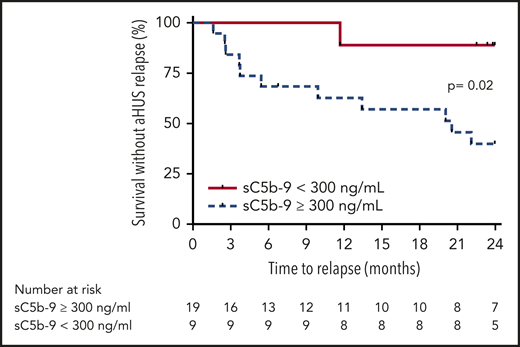
However, increased sC5b-9 plasma level at eculizumab discontinuation was associated with higher risk of aHUS relapse in the entire cohort (Figure 5) and in the subpopulation of carriers of rare variants in complement genes (Figure 6) by log-rank test. Therefore, we performed a second multivariable analysis that included level of sC5b-9 and all other parameters, except presence of a complement gene variant. In this analysis, plasma sC5b-9 ≥300 ng/mL at inclusion was independently associated with aHUS relapse after treatment discontinuation (OR, 20.96; 95% CI, 1.76-250.12; Table 4).
Probability of survival without aHUS relapse after eculizumab discontinuation according to level of sC5b-9 at inclusion in the entire cohort. Risk of relapse was higher in patients with elevated sC5b-9 at inclusion compared with those with normal sC5b-9 levels (P = .02 by log-rank test).
Probability of survival without aHUS relapse after eculizumab discontinuation according to level of sC5b-9 at inclusion in the entire cohort. Risk of relapse was higher in patients with elevated sC5b-9 at inclusion compared with those with normal sC5b-9 levels (P = .02 by log-rank test).
Probability of survival without aHUS relapse after eculizumab discontinuation according to the level of sC5b-9 at inclusion in patients with detected rare variants in complement genes. Risk of relapse was higher in patients with elevated sC5b-9 at inclusion compared with those with normal sC5b-9 levels (P = .02 by log-rank test).
Probability of survival without aHUS relapse after eculizumab discontinuation according to the level of sC5b-9 at inclusion in patients with detected rare variants in complement genes. Risk of relapse was higher in patients with elevated sC5b-9 at inclusion compared with those with normal sC5b-9 levels (P = .02 by log-rank test).
Eculizumab was resumed in all relapsing patients within 7 days of relapse, except in patient 13 (Table 2), in whom serum creatinine and urinary protein/creatinine ratio gradually increased over 3 months before eculizumab was restarted. At last follow-up, serum creatinine levels and eGFRs did not differ between relapsing and nonrelapsing patients (Table 3). Urinary protein/creatinine ratio at last follow-up was higher in relapsing compared with nonrelapsing adults (0.12 vs 0.04 g/mmol; P = .004), but this ratio tended to be higher at eculizumab discontinuation in the first group compared with the second (Table 3). In paired analysis, eGFR and urinary protein/creatinine ratio at eculizumab discontinuation and at last follow-up did not significantly differ between relapsing and nonrelapsing patients (P = .87 and .71 by Wilcoxon paired test, respectively).
Among the 13 patients who relapsed and restarted eculizumab, 11 regained their baseline serum creatinine level and eGFR as early as 3 months after the restart (Table 2). The 2 remaining patients had a worsening of their preexisting CKD. The first patient was a 44-year-old man (patient 11; Table 2); he carried a pathogenic variant in the MCP gene and had stage 4 CKD (eGFR, 27 mL/min/1.73 m2) after 2 previous aHUS episodes treated with eculizumab (for 3 and 5 months, respectively). Three months after eculizumab discontinuation, he experienced aHUS relapse; eculizumab was resumed for 3 months, but eGFR decreased to 19 mL per minute per 1.73 m2. Subsequently, he progressed to stage 5 CKD and underwent successful kidney transplantation without eculizumab prophylaxis. aHUS did not recur after a 2-year follow-up. The second patient was a 56-year-old woman (patient 13; Table 2); she carried a pathogenic CFH variant and had stage 3a CKD at inclusion in the study. Twenty-two months after eculizumab discontinuation, she had a gradual increase over 3 months in serum creatinine (from 101-130 to 232 μmol/L) and urinary protein/creatinine ratio (from 0.02 to 1.06 g/mmol), before eculizumab was restarted. At last follow-up, her eGFR was 29 mL per minute per 1.73 m2.
During the study, included children and adults did not require eculizumab treatment for a median time of 23.6 months (range, 5.4-24 months) and 24 months (range, 1.6-24 months), respectively. The total saved cost of medication (excluding infusion-related costs) was estimated at ∼32.000.000 euros.
Discussion
This prospective study, the first reported in children and adults with aHUS, shows that the risk of aHUS relapse after eculizumab discontinuation is predominantly determined by presence or absence of a rare variant in a complement gene. It clearly indicates that eculizumab discontinuation is feasible and safe in children and adults with aHUS and no detected complement gene variant. In this subset of patients, the risk of aHUS relapse after treatment cessation was <5%. This finding confirms, from a prospective perspective, previously reported data from small retrospective series,12,15-17 which included a proportion of patients with HUS and associated conditions (Table 5). Therefore, in a patient with no detected variant, aHUS relapse should prompt a reassessment of complement genetic results and even of aHUS diagnosis, because the single relapsing patient from the present study with no complement gene variant was subsequently diagnosed with hereditary ADAMTS13 deficiency. If this patient is excluded, the risk of relapse in patients with no complement gene variant is 0 in this prospective study.
Rate of aHUS relapse after eculizumab discontinuation in 4 previously published retrospective series from Europe and the United States and in Present Prospective Study
| . | n (%) . | |||||
|---|---|---|---|---|---|---|
| Ardissino et al11 (2014) . | Fakhouri et al12 (2016) . | Wijnsma et al13 (2017) . | Merrill et al17 (2017) . | Present study . | Total . | |
| Patients with complementgene screening | 16 | 38 | 18* | 13† | 55 | 140 |
| Adults | 8 | 29 | 14 | 13 | 36 | 100 |
| Children | 8 | 9 | 4 | 0 | 19 | 40 |
| Duration of follow-up‡ | NS | |||||
| Median | 13.1 mo | 22 mo | 239 d | 19.8 mo§ | ||
| Range | 0.4-40 | 5-43 | 0-1390 | 5.4-24 | ||
| Patients with no variant and no anti–factor H Ab | 5 | 16 | 4 | 8‖ | 23 | 56 |
| Relapse rate | 0 | 0 | 0 | 1 (13) | 1 (4) | 2 (3.5)¶ |
| Patients with rare variants (MAF <0.1%) | 7 | 21 | 13 | 5 | 28 | 74 |
| Relapse rate | 3 (43) | 12 (57) | 5 (38) | 2 (40) | 12 (43) | 34 (46) |
| Patients with rare pathogenic variant | 4 | 18 | 9 | 2 | 26 | 59 |
| Relapse rate | 3 (75) | 10 (56) | 3 (33) | 2 (100) | 10 (38) | 28 (47) |
| Patients with CFH rare variant | 2 | 11 | 7 | 2 | 6 | 28 |
| P/LP | 2 | 9 | 4 | 1 | 6 | 22 |
| VUS | 0 | 2 | 3 | 1 | 0 | 6 |
| Relapse rate | 2 (100) | 8 (72) | 4 (57) | 1 (50) | 3 (50) | 18 (64) |
| P/LP | 2 | 7 | 2 | 1 | 3 | 15 |
| VUS | 0 | 1 | 2 | 0 | 0 | 3 |
| Patients with MCP rare variant | 2 | 8 | 0 | 2 | 12 | 24 |
| P/LP | 1 | 7 | 1 | 12 | 21 | |
| VUS | 1 | 1 | 1 | 0 | 3 | |
| Relapse rate | 0 | 3 (37) | — | 0 | 6 (50) | 9 (37) |
| P/LP | 3 | 6 | 9 | |||
| VUS | 0 | 0 | 0 | |||
| Patients with CFI rare variant | 3 | 2 | 1 | 0 | 7 | 13 |
| P/LP | 1 | 2 | 0 | 6 | 9 | |
| VUS | 2 | 0 | 1 | 1 | 4 | |
| Relapse rate | 1 (33) | 0 | 0 | — | 2 (29) | 3 (23) |
| P/LP | 1 | 0 | 1 | 2 | ||
| VUS | 0 | 0 | 1 | 1 | ||
| Patients with C3 rare variant | 0 | 1 | 4 | 0 | 2 | 7 |
| P/LP | 0 | 4 | 2 | 6 | ||
| VUS | 1 | 0 | 0 | 1 | ||
| Relapse rate | 0 | 1 (25) | — | 0 | 1 (14) | |
| P/LP | 1 | 1 | ||||
| VUS | 0 | 0 | ||||
| Patients with CFB rare variant | 0 | 0 | 1 | 1 | 0 | 2 |
| P/LP | 1 | 0 | 1 | |||
| VUS | 0 | 1 | 1 | |||
| Relapse rate | — | — | 0 | 1 (100) | 1 (50) | |
| P/LP | 0 | 0 | ||||
| VUS | 1 | 1 | ||||
| Patients with anti–factor H Ab | 4# | 1 | 1 | 0 | 4 | 10 |
| Relapse rate | 2 (50) | 0 | 0 | 0 | 2 (20) | |
| . | n (%) . | |||||
|---|---|---|---|---|---|---|
| Ardissino et al11 (2014) . | Fakhouri et al12 (2016) . | Wijnsma et al13 (2017) . | Merrill et al17 (2017) . | Present study . | Total . | |
| Patients with complementgene screening | 16 | 38 | 18* | 13† | 55 | 140 |
| Adults | 8 | 29 | 14 | 13 | 36 | 100 |
| Children | 8 | 9 | 4 | 0 | 19 | 40 |
| Duration of follow-up‡ | NS | |||||
| Median | 13.1 mo | 22 mo | 239 d | 19.8 mo§ | ||
| Range | 0.4-40 | 5-43 | 0-1390 | 5.4-24 | ||
| Patients with no variant and no anti–factor H Ab | 5 | 16 | 4 | 8‖ | 23 | 56 |
| Relapse rate | 0 | 0 | 0 | 1 (13) | 1 (4) | 2 (3.5)¶ |
| Patients with rare variants (MAF <0.1%) | 7 | 21 | 13 | 5 | 28 | 74 |
| Relapse rate | 3 (43) | 12 (57) | 5 (38) | 2 (40) | 12 (43) | 34 (46) |
| Patients with rare pathogenic variant | 4 | 18 | 9 | 2 | 26 | 59 |
| Relapse rate | 3 (75) | 10 (56) | 3 (33) | 2 (100) | 10 (38) | 28 (47) |
| Patients with CFH rare variant | 2 | 11 | 7 | 2 | 6 | 28 |
| P/LP | 2 | 9 | 4 | 1 | 6 | 22 |
| VUS | 0 | 2 | 3 | 1 | 0 | 6 |
| Relapse rate | 2 (100) | 8 (72) | 4 (57) | 1 (50) | 3 (50) | 18 (64) |
| P/LP | 2 | 7 | 2 | 1 | 3 | 15 |
| VUS | 0 | 1 | 2 | 0 | 0 | 3 |
| Patients with MCP rare variant | 2 | 8 | 0 | 2 | 12 | 24 |
| P/LP | 1 | 7 | 1 | 12 | 21 | |
| VUS | 1 | 1 | 1 | 0 | 3 | |
| Relapse rate | 0 | 3 (37) | — | 0 | 6 (50) | 9 (37) |
| P/LP | 3 | 6 | 9 | |||
| VUS | 0 | 0 | 0 | |||
| Patients with CFI rare variant | 3 | 2 | 1 | 0 | 7 | 13 |
| P/LP | 1 | 2 | 0 | 6 | 9 | |
| VUS | 2 | 0 | 1 | 1 | 4 | |
| Relapse rate | 1 (33) | 0 | 0 | — | 2 (29) | 3 (23) |
| P/LP | 1 | 0 | 1 | 2 | ||
| VUS | 0 | 0 | 1 | 1 | ||
| Patients with C3 rare variant | 0 | 1 | 4 | 0 | 2 | 7 |
| P/LP | 0 | 4 | 2 | 6 | ||
| VUS | 1 | 0 | 0 | 1 | ||
| Relapse rate | 0 | 1 (25) | — | 0 | 1 (14) | |
| P/LP | 1 | 1 | ||||
| VUS | 0 | 0 | ||||
| Patients with CFB rare variant | 0 | 0 | 1 | 1 | 0 | 2 |
| P/LP | 1 | 0 | 1 | |||
| VUS | 0 | 1 | 1 | |||
| Relapse rate | — | — | 0 | 1 (100) | 1 (50) | |
| P/LP | 0 | 0 | ||||
| VUS | 1 | 1 | ||||
| Patients with anti–factor H Ab | 4# | 1 | 1 | 0 | 4 | 10 |
| Relapse rate | 2 (50) | 0 | 0 | 0 | 2 (20) | |
Complement gene variants were classified as pathogenic, likely pathogenic, or of unknown significance according to recommendations published by Richards et al.23
Ab, antibody; MAF, minor allele frequency; NS, not specified; P/LP, pathogenic/likely pathogenic; VUS, variant of unknown significance.
Including 4 renal transplant recipients.
The study included 6 patients with HUS and coexisting diseases/conditions: liver transplantation (n = 1), quinine use (n = 1), possible scleroderma (n = 1), inflammatory bowel disease (n = 1), and polymyositis-scleroderma overlap (n = 1). Two additional patients had malignant hypertension, including 1 with TMA attributed to accelerated primary hypertension. The 3 HUS relapses after eculizumab discontinuation occurred in the settings of active inflammatory bowel disease (n = 1), malignant hypertension with nonadherence to antihypertensive treatment (n = 1), and liver transplantation with medication nonadherence (n = 1).
Time to relapse or last follow-up.
Mean.
Two patients had heterozygous ADAMTS13 variants (1 relapsed), and another had heterozygous DGKe variant.
The 2 HUS relapses after eculizumab discontinuation in patients without identified rare variants occurred in the settings of liver transplantation with medication nonadherence (n = 1) and late discovery of complete ADAMTS13 deficiency (n = 1).
One patient with anti–factor H antibodies carried CFH gene variant. Titer of the autoantibodies was not specified.
In contrast, in carriers of complement gene variants, the risk of aHUS relapse is significant and estimated at ∼50% in patients with the most frequently encountered variants, in the CFH and MCP genes. This is in keeping with previous results from small retrospective studies (Table 5). Of note, the proportion of patients with CFH variants included in the present study was rather low (11%) compared with previous cohorts of aHUS patients.4,5,18 This may be due to the reluctance of clinicians and patients to discontinue treatment, based on the high risk of relapse reported in previous published retrospective series (Table 5). Nevertheless, the decision of whether to discontinue eculizumab in patients with complement gene variants should be discussed with each patient individually following a shared decision model. However, the present study identifies several features, in addition to complement genetics, that may help in decision making.
First, female patients seemed be at increased risk of relapse compared with male patients. There is no clear explanation for this finding, and none of the relapses during this study were precipitated by pregnancy, a recognized high-risk period for aHUS.19 Second, elevated sC5b-9 level at eculizumab discontinuation was independently associated with risk of aHUS relapse in multivariable analysis. This biomarker should prove helpful in the assessment of aHUS relapse risk, particularly in the subset of patients with complement gene variants (Table 4). sC5b-9 has been shown to remain detectable in a significant proportion of patients despite optimal C5 blockade, for unclear reasons that may involve cleavage of C5 via C5 convertase-independent pathways.20-22 Similarly, there is no clear explanation for the persistence of not only detectable but also increased sC5b-9 plasma levels with eculizumab in a significant proportion of included patients. Nevertheless, the present study provides the first evidence of the relevance of a simple biomarker, sC5b-9, in the assessment of the risk of aHUS relapse after treatment cessation in patients with constitutional complement dysregulation. However, these results should be interpreted with caution because of the limited number of patients included, and the predictive value of this biomarker warrants further assessment in larger cohorts of aHUS patients.
Furthermore, although in the present study the severity of acute aHUS (dialysis requirement, extrarenal manifestations) was not associated with increased risk of aHUS relapse after treatment cessation, significant residual CKD (including proteinuria without decrease in eGFR) in an eculizumab-treated aHUS patient should encourage caution in treatment discontinuation. It remains unclear whether kidney vascular damage after an initial aHUS flare increases the risk of additional relapses. Nevertheless, after aHUS relapse in a patient with significant CKD, even an early restart of eculizumab does not guarantee optimal recovery of kidney function. This is particularly true for adults in whom renal function recovery with eculizumab is less optimal compared with children, as exemplified by the present as well as previous reports.2,9 Indeed, 2 adult patients in our study with residual significant CKD did not recover their baseline kidney function after relapse, including the 1 patient who progressed to stage 5 CKD. In the other patient, restart of eculizumab treatment was delayed by 3 months, because the initial presentation of aHUS relapse was isolated proteinuria, and this delay most probably contributed to the nonoptimal recovery of renal function. In all remaining relapsing patients, there was no additional significant kidney damage after recovery from aHUS relapse.
However, it needs to be stressed that patients from the present study, except 1, had received eculizumab treatment for at least 3 to 6 months and achieved maximal kidney function recovery before inclusion. They were closely monitored after treatment cessation throughout follow-up (particularly during infectious episodes), and of note, some patients experienced aHUS relapse >18 months after treatment cessation. Eculizumab was restarted in all but 1 patient within a week of aHUS relapse diagnosis. A patient’s strict adhesion to monitoring and the guarantee of an early restart of treatment in the case of a complete or incomplete picture of aHUS relapse are absolute prerequisites for eculizumab discontinuation. Furthermore, relapsing patients were followed for a relatively limited period, and the long-term impact of (repeated) relapses on kidney function remains to be assessed. This applies particularly to carriers of MCP variants, who are assumed to have rather good long-term renal outcomes and thus are usually treated with eculizumab for a limited period (3 months) at each relapse.
In conclusion, this prospective study indicates that a strategy of eculizumab discontinuation in aHUS patients based on complement genetics is reasonable and safe. Such a strategy undoubtedly will improve the management and quality of life of a sizeable proportion of aHUS patients. It also allows for a significant reduction in health costs (32 million euros over 2 years in the present study) and optimal use of an innovative and expensive treatment.
E-mail the corresponding author for original data.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for their participation in the study. The authors are also grateful to Anne Chiffoleau, Elodie Faurel-Paul, Marion Rigot, Edith Faillet, and Stéphanie Molle from the Direction de la Recherche at Centre Hospitalier Universitaire de Nantes for their help in conducting this study.
This study was supported by the Programme Hospitalier de Recherche Clinique (EUDRACT #2015-000678-37) by the Fondation du Rein (Prix 2012 FDR) (V.F.-B.) and the Association pour l’Information et la Recherche dans les Maladies Rénales Génétiques.
Authorship
Contribution: F.F., C.L., and V.F.-B. designed the study; all authors contributed to the recruitment of patients in the study and to their management; A.L.T. performed the statistical analysis; F.F. and V.F.-B. analyzed the data and drafted the manuscript; and all authors read, commented on, and revised the manuscript before approval.
Conflict-of-interest disclosure: F.F. has received consultancy and/or speaker honoraria from Roche, Alexion, Apellis, Achillion, Novartis, and Alnylam. F.P. has received speaker fees from Alexion and Sanofi. Y.D. has received lecture fees from Alexion and honoraria for advisory board membership from Sanofi-Ablynx. E.R. reports grants and personal fees from Alexion Pharmaceuticals. O.B. has received consultancy honoraria from Alexion. M.L.Q. reports personal fees from Novartis and Alexion. C.L. has received consultancy fees for advisory boards from Alexion, Roche, Inmunova, and Eligo Bioscience. V.F.-B. has received fees from Alexion Pharmaceuticals, Roche, and Apellis for invited lectures and/or board membership and is the recipient of a research grant from Alexion Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Fadi Fakhouri, Service of Nephrology and Hypertension, Department of Medicine, Lausanne University Hospital, Lausanne, Switzerland; e-mail: fadi.fakhouri@unil.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal