Key Points
Increased expression and activity of β-1,4-galactosyltransferase1 is observed in megakaryocytes from MPNs with high allele burden.
Highly galactosylated platelets in MPNs may promote increased hepatic thrombopoietin synthesis that sustains the aberrant megakaryopoiesis.
Abstract
Aberrant megakaryopoiesis is a hallmark of the myeloproliferative neoplasms (MPNs), a group of clonal hematological malignancies originating from hematopoietic stem cells, leading to an increase in mature blood cells in the peripheral blood. Sialylated derivatives of the glycan structure β4-N-acetyllactosamine (Galβ1,4GlcNAc or type-2 LacNAc, hereafter referred to as LacNAc) regulate platelet life span, hepatic thrombopoietin (TPO) production, and thrombopoiesis. We found increased TPO plasma levels in MPNs with high allele burden of the mutated clones. Remarkably, platelets isolated from MPNs had a significant increase in LacNAc expression that correlated with the high allele burden regardless of the underlying identified mutation. Megakaryocytes derived in vitro from these patients showed an increased expression of the B4GALT1 gene encoding β-1,4-galactosyltransferase 1 (β4GalT1). Consistently, megakaryocytes from MPN showed increased LacNAc expression relative to healthy controls, which was counteracted by the treatment with a Janus kinase 1/2 inhibitor. Altered expression of B4GALT1 in mutant megakaryocytes can lead to the production of platelets with aberrant galactosylation, which in turn promote hepatic TPO synthesis regardless of platelet mass. Our findings provide a new paradigm for understanding aberrant megakaryopoiesis in MPNs and identify β4GalT1 as a potential actionable target for therapy.
Introduction
Philadelphia chromosome–negative myeloproliferative neoplasms (MPNs) are a group of clonal hematopoietic malignancies that are caused by acquired driver mutations in hematopoietic stem cells (HSCs), including activating mutations in the Janus kinase 2 (JAK2) gene, activating mutations in the MPL gene, and mutations of CALR.1 Approximately 10% of patients do not carry any of these mutations. The different genotypes share a common biochemical phenotype related to an abnormal constitutive activation of JAK/STAT signaling that promotes neoplastic proliferation of megakaryocytes in the bone marrow.2 The aberrant megakaryopoiesis is thought to exacerbate the progression of MPNs, but the mechanisms remain elusive.
One unanswered question is the regulation of thrombopoietin (TPO) production in MPNs, whose levels do not correlate with platelet and megakaryocyte mass.3-5 Under physiologic conditions, hepatic expression of TPO is regulated by platelets that expose galactose moieties to the Ashwell-Morell receptor (AMR).6 Galactosylation is also relevant for thrombopoiesis. The β1-4galactosyl transferase (βGalT) 1-7 subfamily consists of at least 7 members.7 However, only β4GalT1 and β4GalT2 synthesize Galβ1,4GlcNAc (LacNAc) on N-linked glycans. While alterations in the activity of some of the β4GalT subfamily members have been associated with various diseases,8,9 our recent data show that deletion of the B4GALT1 leads to severe thrombocytopenia and impaired HSC homeostasis.10 Additionally, in megakaryocytes B4GALT1 expression is regulated via JAK signaling to control glycan expression on proteins.10,11 Thus, galactosylation regulated by JAK plays an important role in megakaryopoiesis.
β4GalT1 regulates many biological events, including embryogenesis and morphogenesis, cell-cell and cell-matrix interactions, cell migration, homing, and engraftment of HSCs to the bone marrow.12-15 Moreover, B4GALT1 is overexpressed in pathological processes, including inflammation and proliferation of cancer cells, making it conceivable to target the enzyme in anticancer therapies.16-18
Despite this knowledge, platelet and megakaryocyte galactosylation has not been studied in MPNs. Here, we show that B4GALT1 is highly expressed in human-derived megakaryocytes and platelets isolated from MPNs and that its expression profile correlates with the mutated clone allele burden and TPO plasma concentration.
Study design
Sampling and analysis
Blood samples from healthy controls (HCs) and MPNs (supplemental Table 1, available on the Blood Web site) were collected in citric acid-citrate-dextrose after obtaining informed consent in accordance with the local ethical committee and the principles of the Declaration of Helsinki. None of the patients were receiving disease-modifying therapy at the time of their enrollment in the study. Platelets were obtained from platelet-rich plasma,19,20 while CD34+ cells were obtained by immunomagnetic sorting and differentiated into megakaryocytes in the presence of 10 ng/mL TPO.21
For the analysis of basal B4GALT1 and LacNAc expression, MPN megakaryocytes at day 10 of culture were cytokine starved for 3 days and treated or not with 100 nM of the JAK1/2 inhibitor ruxolitinib. For the analysis of LacNAc expression induced by pharmacologically activated JAK/STAT pathway, HC megakaryocytes were cultured with 100 ng/mL TPO for 3 days and treated or not with ruxolitinib.
A detailed protocol regarding quantification of TPO plasma levels, platelet isolation, immunoblotting, megakaryocyte culture, quantitative real-time polymerase chain reaction, and immunofluorescence analysis is described in supplemental Materials and methods.
Statistical analysis
Values are expressed as mean ± standard deviation (SD). The Student t test was used to analyze experiments. A value of P < .05 was considered statistically significant. All experiments were independently repeated ≥3 times, unless specified otherwise.
Results and discussion
We measured circulating TPO plasma levels in HCs and MPN patients. The median value in diseased samples was 107 pg/mL (range, 65-426 pg/mL), significantly higher than HCs (median, 84 pg/mL; range, 70-95 pg/mL). We found a statistically significant correlation between circulating TPO levels and allele burden of the main driver mutations (JAK2V617F and CALR). Patients having >30% allele burden showed the highest TPO concentrations (Figure 1A). A significant increased platelet mass was observed in MPNs regardless of the type of mutation (supplemental Table 1), which did not correlate with TPO concentration, confirming previous evidence.3-5
Increased TPO plasma levels and platelet galactosylation in MPNs. (A) TPO plasma levels were measured in samples from patients with MPNs with different driver mutations and percentages of allelic burden. The percentage of allelic burden correlated with TPO concentration (n = 38, P < .0001, R2 = 0.6). (B) Platelets that expose galactose are recognized by the hepatic AMR to regulate hepatic TPO production (LacNAc; N-acetylglucosamine [GlcNAc]). (C) Flow cytometry analysis of expression of GpIbα and GpIIb in platelets from HCs and MPN patients. (D) HC and MPN platelets were lysed, and total lysates were subjected to SDS-PAGE and probed with ECL (i) and RCA (ii) in order to evaluate galactosylation status. GpIbα, GPIIIa, and β-actin have been used as loading controls. (E-F) Densitometric analysis of ECL and RCA signal in MPNs (n = 12) relative to HCs (n = 8). Data are presented as mean ± SD (*P < .05). FSC, forward scatter; SSC, side scatter.
Increased TPO plasma levels and platelet galactosylation in MPNs. (A) TPO plasma levels were measured in samples from patients with MPNs with different driver mutations and percentages of allelic burden. The percentage of allelic burden correlated with TPO concentration (n = 38, P < .0001, R2 = 0.6). (B) Platelets that expose galactose are recognized by the hepatic AMR to regulate hepatic TPO production (LacNAc; N-acetylglucosamine [GlcNAc]). (C) Flow cytometry analysis of expression of GpIbα and GpIIb in platelets from HCs and MPN patients. (D) HC and MPN platelets were lysed, and total lysates were subjected to SDS-PAGE and probed with ECL (i) and RCA (ii) in order to evaluate galactosylation status. GpIbα, GPIIIa, and β-actin have been used as loading controls. (E-F) Densitometric analysis of ECL and RCA signal in MPNs (n = 12) relative to HCs (n = 8). Data are presented as mean ± SD (*P < .05). FSC, forward scatter; SSC, side scatter.
Since TPO production in the liver is promoted by the activation of JAK/STAT signaling through the binding of the platelet galactose to the AMR in hepatocytes6 (Figure 1B), we asked whether platelets from MPNs have an increased terminal LacNAc on their surface. We isolated platelets from peripheral blood of HCs and MPNs. The purity of the population was ∼98% in both groups (Figure 1C). Erythrina cristagalli lectin (ECL) and Ricinus communis agglutinin I (RCA) were used to determine the expression of the terminal LacNAc in protein lysates.6 Platelets from patients had a significant increase in LacNAc expression regardless of the underlying identified mutation (Figure 1D-E). The signal from patients with >30% allele burden was significantly higher than those with <30% allele burden (Figure 1F). Increased galactosylation was also confirmed in patients with MPL mutation or a triple-negative genotype (supplemental Figure 1). No significant differences where observed in the expression of platelet markers, even when segregated by the allele burden (supplemental Figure 2). Whether sialic acid levels are altered in MPNs is currently unclear.
We have recently demonstrated that TPO increases the expression of LacNAc by megakaryocytes through activation of JAK/STAT signaling, which in turn promotes expression of B4GALT1.10 We reasoned that the platelet galactosylation observed in MPN patients with high allele burden could be due to increased B4GALT1 expression and β4GalT1 activity. Megakaryocytes were differentiated from CD34+ cells isolated from the peripheral blood of HCs and MPNs with >30% allele burden. Consistent with previous findings,22 samples from MPNs demonstrated increased megakaryocyte proliferation but comparable expression of markers with respect to HCs (Figure 2A-B). Mature megakaryocytes were cytokine starved in order to analyze B4GALT1 expression in the absence of exogenous stimuli, including TPO. Results demonstrated that mutated samples present a significant increased expression of the gene with respect to HCs (Figure 2C). Consistently, megakaryocytes from MPNs showed increased LacNAc expression relative to HCs, as determined by immunofluorescence using ECL lectin (Figure 2D). When treated with Ruxolitinib, a JAK1/2 inhibitor, MPN megakaryocytes demonstrated a decreased LacNAc expression (Figure 2Ei). Consistently, when megakaryocytes from HCs were treated with a high dose of TPO (100 ng/mL) in order to stimulate the JAK/STAT pathway, a strong reduction of LacNAc expression was observed in the presence of ruxolitinib as compared with the sample treated with TPO only (Figure 2Eii), supporting the evidence that megakaryocyte galactosylation is dependent on JAK/STAT activation.
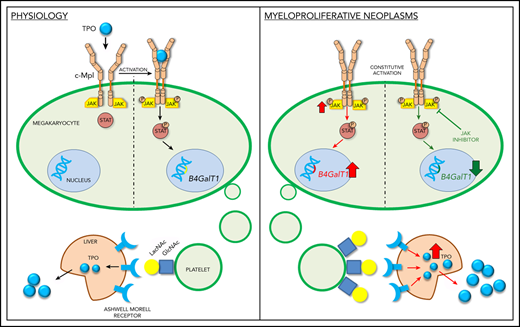
Megakaryocytes from MPNs express increased B4GALT1 under the control of JAK/STAT pathway. Megakaryocytes were differentiated from human peripheral blood progenitors from HCs and MPN patients. (A) Representative immunofluorescence staining of GpIIIa (green, GpIIIa; blue, nuclei; scale bar, 25 µm). (B) Percentage of GpIIb+GpIbα+ megakaryocytes (MKs) at the end of the culture. Data are presented as mean ± SD (n = 8; P, not significant). (C) B4GALT1 expression differentiated from HCs and patients. Data are presented as mean ± SD (n = 3; *P < .05). (D) Immunofluorescence of megakaryocytes differentiated from HC and patients with MPN using ECL (red, ECL; blue, nuclei; scale bar, 20 μm). The quantification of ECL fluorescence in megakaryocytes is reported. Data are presented as mean ± SD (n = 8; *P < .001). (E) MPN megakaryocytes were cytokine starved and treated or not with ruxolitinib (i). HC megakaryocytes were cultured with a high dose of TPO and treated or not with ruxolitinib (ii). Two MPNs (JAK2 and MPL) and 3 HCs were tested with comparable results. Here, we present the representative western blot analysis of total lysates from the JAK2-mutated patient and 1 HC, probed with ECL in order to evaluate the galactosylation status. GpIbα, GpIIIa, and β-actin were used as loading controls. (F) Diagram of how B4GALT1 regulates megakaryocyte and platelet galactosylation in physiology and disease. In HCs (left panel), B4GALT1 is expressed under control of TPO. Megakaryocytes produce and release platelets into the bloodstream that expose galactose becoming ligands for the AMRs to promote TPO production by the liver. In MPNs (right panel), constitutive activation of JAK/STAT signaling determines an increased expression of B4GALT1 resulting in the production of platelets that are highly galactosylated. This can stimulate hepatocytes to release high amounts of TPO into bloodstream.
Megakaryocytes from MPNs express increased B4GALT1 under the control of JAK/STAT pathway. Megakaryocytes were differentiated from human peripheral blood progenitors from HCs and MPN patients. (A) Representative immunofluorescence staining of GpIIIa (green, GpIIIa; blue, nuclei; scale bar, 25 µm). (B) Percentage of GpIIb+GpIbα+ megakaryocytes (MKs) at the end of the culture. Data are presented as mean ± SD (n = 8; P, not significant). (C) B4GALT1 expression differentiated from HCs and patients. Data are presented as mean ± SD (n = 3; *P < .05). (D) Immunofluorescence of megakaryocytes differentiated from HC and patients with MPN using ECL (red, ECL; blue, nuclei; scale bar, 20 μm). The quantification of ECL fluorescence in megakaryocytes is reported. Data are presented as mean ± SD (n = 8; *P < .001). (E) MPN megakaryocytes were cytokine starved and treated or not with ruxolitinib (i). HC megakaryocytes were cultured with a high dose of TPO and treated or not with ruxolitinib (ii). Two MPNs (JAK2 and MPL) and 3 HCs were tested with comparable results. Here, we present the representative western blot analysis of total lysates from the JAK2-mutated patient and 1 HC, probed with ECL in order to evaluate the galactosylation status. GpIbα, GpIIIa, and β-actin were used as loading controls. (F) Diagram of how B4GALT1 regulates megakaryocyte and platelet galactosylation in physiology and disease. In HCs (left panel), B4GALT1 is expressed under control of TPO. Megakaryocytes produce and release platelets into the bloodstream that expose galactose becoming ligands for the AMRs to promote TPO production by the liver. In MPNs (right panel), constitutive activation of JAK/STAT signaling determines an increased expression of B4GALT1 resulting in the production of platelets that are highly galactosylated. This can stimulate hepatocytes to release high amounts of TPO into bloodstream.
Together, these data show that constitutive activation of JAK/STAT in MPN megakaryocytes leads to increased B4GALT1 expression and consequently increased LacNAc exposure in megakaryocytes and platelets, which is associated with increased TPO levels in blood. These alterations may account for a deregulated loop where MPN megakaryocytes produce platelets with increased exposure of terminal galactose that promotes increased hepatic TPO synthesis (Figure 2F). To confirm this hypothesis, thrombocytopenia is a common adverse event of treatment with JAK1/2 inhibitors.23,24 Thus, constitutive activation of the JAK/STAT pathway and altered regulation of TPO secretion by the liver may contribute to the aberrant megakaryopoiesis observed in MPNs. Hence, we speculate that altered regulation of the B4GALT1 promoter may be associated with increased progression and severity in MPNs, and may be considered a novel therapeutic target for this complex disease. This study is focused on the role of galactosylation and B4GALT1. The enzyme MGAT3 (β1,4-mannosyl-glycoprotein 4-β-N-acetylglucosaminyltransferase) catalyzes addition of a bisecting GlcNAc residue to N-linked glycans on glycoproteins (a prerequisite for galactose addition), which has a suppressive effect on several types of cancer.25 Future studies need to determine if MGAT3 or sialyltransferases are affected in patients with MPNs.
For original data, please, contact the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This paper was supported by the Associazione Italiana per la Ricerca sul Cancro (IG 2016 18700 and AIRC 5x1000 call “Metastatic disease: the key unmet need in oncology” to MYNERVA [MYeloid NEoplasms Research Venture Airc] project #21267), the Cariplo Foundation (2013-0717 and 2017-0920), National Institutes of Health (NIH)/National Institute of Biomedical Imaging and Bioengineering grant R01 EB016041-02, NIH/National Heart, Lung, and Blood Institute grants R01 HL134829, R01 HL089224, and K12 HL141954, and the Progetti di Ricerca di Rilevante Interesse Nazionale (2017Z5LR5Z).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: C.A.D.B. designed and performed research, acquired data, analyzed data, and wrote the manuscript; S.G. and V.A. designed and performed research, acquired data, analyzed data, and edited the manuscript; V.R. provided patient samples, analyzed data, and edited the manuscript; K.M.H. designed research, analyzed data, provided reagents, and wrote the manuscript; and A.B. supervised the project, designed research, acquired data, analyzed data, provided reagents, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandra Balduini, Department of Molecular Medicine, University of Pavia, Via Forlanini 6, 27100, Pavia, Italy; e-mail: alessandra.balduini@unipv.it.

![Increased TPO plasma levels and platelet galactosylation in MPNs. (A) TPO plasma levels were measured in samples from patients with MPNs with different driver mutations and percentages of allelic burden. The percentage of allelic burden correlated with TPO concentration (n = 38, P < .0001, R2 = 0.6). (B) Platelets that expose galactose are recognized by the hepatic AMR to regulate hepatic TPO production (LacNAc; N-acetylglucosamine [GlcNAc]). (C) Flow cytometry analysis of expression of GpIbα and GpIIb in platelets from HCs and MPN patients. (D) HC and MPN platelets were lysed, and total lysates were subjected to SDS-PAGE and probed with ECL (i) and RCA (ii) in order to evaluate galactosylation status. GpIbα, GPIIIa, and β-actin have been used as loading controls. (E-F) Densitometric analysis of ECL and RCA signal in MPNs (n = 12) relative to HCs (n = 8). Data are presented as mean ± SD (*P < .05). FSC, forward scatter; SSC, side scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/137/15/10.1182_blood.2020007265/1/m_bloodbld2020007265r1f1.png?Expires=1769084586&Signature=mziy6GV-kBR7jOk2gjAlbBcOI04G7BcoNyHXa6h3XFcptFAFIMZlaim5Amy23fA5~htcaGP7CcUHEmxpXLjkIbG2OMnO2HiJb0htf0JI7K9w5T3m4VbLZ15gy8RDr2D8VdEtgQv8TklKT4ObxdAM7OXYpZBe6LqQRAji53ce6s2F8eZCtLUMOdbwK-GAv3-ROAEoqLa-MUiFPVPkbr1anjDaCMLiV7VtOuKJ-QWy8ERGr6DikF8-UerMloPmbMfQP--rpAM0k4UeZxMoJuazY1-CxHQJLXzGrwtac-YAzKa-HD6juoiGG6cEVjh-Ujsfq2QYA7zDzwHTMwDESrWOcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal