In this issue of Blood, Iacobucci et al1 report a preclinical mouse model of acute erythroid leukemia (AEL) created by CRISPR/Cas9-mediated multiplexed gene editing of hematopoietic stem and progenitor cells (HSPCs). The model was then used to probe the pathogenesis of erythroleukemia and explore potential therapeutic targets to treat AEL.
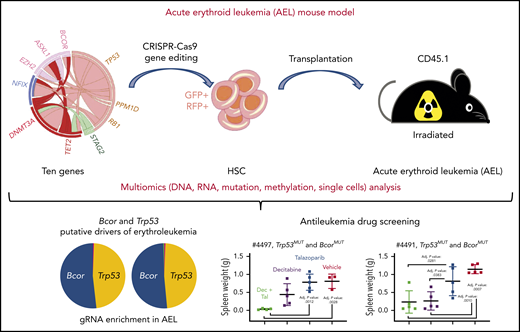
The pipeline used to establish an AEL mouse model. The figure has been adapted from Figure 1B,F, 2C, and 6C-D in the article by Iacobucci et al that begins on page 1628.
The pipeline used to establish an AEL mouse model. The figure has been adapted from Figure 1B,F, 2C, and 6C-D in the article by Iacobucci et al that begins on page 1628.
AEL is a rare subtype of leukemia, characterized by erythroblastic proliferation in the bone marrow, a distinct genetic profile, and poor outcome.2-5 Experimental models of AEL are limited. Multiple genetic mutations are observed in most cases of AEL,6,7 and it is crucial to establish preclinical AEL models to determine the effects of combinatorial mutations on the initiation and progression of AEL. In their study, Iacobucci et al developed a preclinical mouse model of AEL by using the multiplexed CRISPR genome-editing approach, by which the pathogenesis of erythroleukemia through multiomics (DNA, RNA, mutation, methylation, and single-cell analysis) was revealed, and potential therapeutic targets via drug screening with the AEL mouse model were suggested (see figure).
Previous studies have shown that AEL patients often carry complex mutations in known AML-associated oncogenes.6,7 However, how these genes drive cells to leukemia is poorly understood. Based on human AEL mutational hotspots, Iacobucci et al chose 10 genes (Trp53, Tet2, Dnmt3a, Asxl1, Ezh2, Stag2, Bcor, Ppm1d, Rb1, and Nfix) to target in mouse HSPCs with a multiplexed CRISPR/Cas9 genome-editing technology. According to the mutation cooccurrence and/or exclusivity pattern observed in human AEL,6 they further divided the 10 genes into 6 pools. They generated 6 different lentiviral pools of 10 single-guide RNAs against the above 10 genes and transduced them into the mouse lineage–negative HSPCs from Cas9-eGFP mice for subsequent transplant assays. The arising tumors were subjected to pathologic analysis, multiomics analysis (DNA, RNA, methylation profiling, and transcriptomic and mutational evolution analysis at the single-cell level), and finally drug sensitivity screening was performed in vitro and in vivo.
Iacobucci et al found that the combination of mutations in Bcor, Trp53, Dnmt3a, Rb1, and Nfix could specify erythroid phenotype and recapitulate the genomic features of human leukemia. The AEL mouse models were also used for drug screening, and they found that anti-leukemia drug sensitivity was genotype dependent. A PARP inhibitor (talazoparib) and a demethylating agent (decitabine) were effective in Trp53/Bcor–mutant AEL, whereas CDK7/9 inhibitors were effective in Trp53/Bcor/Dnmt3a–mutant AEL.
Thus, these are the first CRISPR-led AEL mouse models to provide definitive evidence that mutations in both Trp53 and Bcor in conjunction with additional events (eg, epigenetic regulators and transcription factors) are associated with fully penetrant AEL in mice, and shedding light, at least in part, on the molecular and cellular features underlying this particular type of leukemia. Notably, there are differences between the gene editing–induced AEL in mice and what Iacobucci et al previously reported for the human disease,6 as well as what has been observed in mouse AEL models previously established by them6 and others.7,8 Recently, AEL has also been induced by enforcing expression of hematopoietic lineage transcription factors7 or in Bcor−/−/Dnmt3a−/−–double-knockout mice.8 In future studies, the use by the authors of Trp53- and Bcor-knockout mice to establish AEL models may better simulate the more common heterozygous mutations in humans and definitively confirm the cooperative roles of Trp53 and Bcor mutations in AEL.
Preclinical studies using these CRISPR-led AEL mouse models and human cell lines offered the opportunity to identify novel therapeutic vulnerabilities that are not associated solely with this leukemia lineage, but are dependent upon leukemia genotype. The engineered models of AEL represent a potential platform for the identification and validation of drugs for improving therapy for patients with AEL. Future work is also needed to verify the sensitivity of such antileukemia drugs in primary human AEL cells and ideally in a human AEL patient-derived xenograft mouse model.
Conflict-of-interest disclosure: The authors declare no competing financial interests.