In this issue of Blood, Shahneh et al reveal important functions of regulatory T cells (Tregs) in thrombus resolution in a mouse model.1 Deep vein thrombosis (DVT) is a major cause of global morbidity and mortality. Despite the use of anticoagulants, vasoactive drugs, and physical clot removal techniques, DVT still causes permanent disability and mortality. Resolving the clot is difficult, and nonresolved clots can cause long-term pathologies, including postthrombotic syndrome and chronic thromboembolic pulmonary hypertension.2
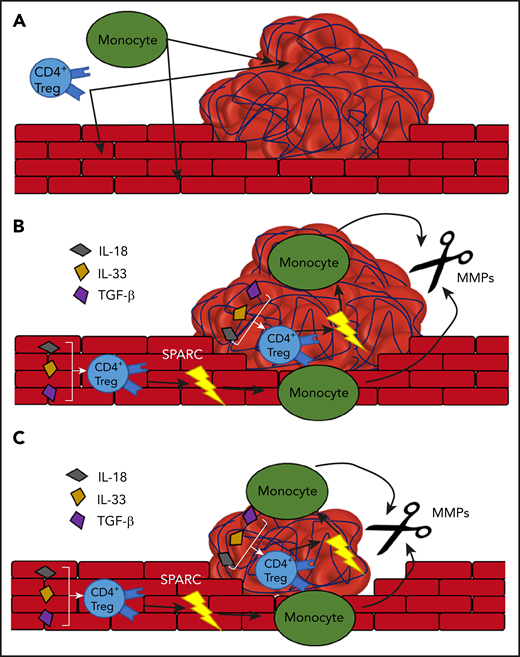
The proposed model of Treg-dependent thrombolysis by monocytes. (A) The formation of a thrombus in the blood vessel attracts monocytes and Tregs. (B) The Tregs express SPARC in an IL-18, IL-33, and TGF-β–dependent manner. SPARC stimulates the release of MMPs from the monocytes. (C) The MMPs degrade the thrombus and thus help with its removal.
The proposed model of Treg-dependent thrombolysis by monocytes. (A) The formation of a thrombus in the blood vessel attracts monocytes and Tregs. (B) The Tregs express SPARC in an IL-18, IL-33, and TGF-β–dependent manner. SPARC stimulates the release of MMPs from the monocytes. (C) The MMPs degrade the thrombus and thus help with its removal.
Over the years, it has become evident that the immune system plays a pivotal role in both thrombus formation and resolution. Although the specific involvement and role of many immune cell subtypes in thrombus formation and resolution have not yet been elucidated, the immunothrombosis field is advancing. For example, monocytes were found to be involved in blood clotting as well as in driving fibrinolysis and clot remodeling.3 Already by 2016, Shahneh et al showed that that effector T cells delay the resolution of thrombosis through interferon-γ and by activating monocytes.4 In that same study, they observed an accumulation of Tregs, a T-cell subset implicated in tissue repair and regeneration,5 in the resolving thrombus. Here, the authors have characterized these thrombus Tregs and elucidate their role in thrombus resolution.1
To induce a thrombus, the authors used a mouse model of partial flow reduction in the inferior vena cava. By isolating immune cells at different time points after the partial occlusion, they showed that Tregs accumulate in the thrombus over time. Clot Tregs were found to be recruited from both the thymus and the periphery and differentiated into an activated, resident phenotype (CD4+FoxP3+CD69+KLF2−). Depletion of these Tregs was shown to impair clot resolution, whereas their expansion by IL2-anti-IL2 complexes facilitated clot resolution. Transcriptional profiling of clot Tregs revealed that clot Tregs are of a special subset, characterized by expression of “secreted protein acidic and rich in cysteines” (SPARC). SPARC is a matrix binding glycoprotein that is involved in many processes, including bone mineralization, cell-matrix interactions, immune cell migration, as well as the production and activity of matrix metalloproteinases (MMPs).6-8
Detailed investigation into the role of SPARC+ Tregs showed that SPARC does not mitigate Treg-mediated regulation of the adaptive immune system. SPARC+ Tregs rather mediate innate immune cell functions and boost MMP production by monocytes, thereby facilitating clot resolution (see figure). The data that Tregs produce SPARC when stimulated with interleukin-18 (IL-18), IL-33, and transforming growth factor-β (TGF-β), without T-cell receptor stimulation, strengthen the hypothesis that SPARC+ Tregs are tissue induced, especially because these cytokines are all associated with the vasculature, where they are produced by the endothelial cells. However, it would be interesting to see whether exposure to TGF-β alone could induce the same SPARC+ phenotype, because TGF-β is known to induce FoxP3+ expression.9 Furthermore, IL-18 and IL-33 are generally seen as proinflammatory, whereas TGF-β, in the context of T cells, is seen as more anti-inflammatory.10
The findings described in this paper are of major importance, with potential therapeutic implications. As the authors have shown in their mouse model, expansion of SPARC+ Tregs in patients with DVT may, as combination therapy with anticoagulants, result in improved clot resolution, thereby preventing chronicity of thrombotic disease. Moreover, SPARC+ Tregs seem to be “unique” to thrombus, and their beneficial effects are limited to 1 thrombotic event, thereby providing a targeted treatment option that likely has no side effects. However, additional research into the distribution and mechanisms of SPARC+ Tregs is needed. The relevance of this Treg subset in human thrombosis needs to be investigated. Furthermore, as of now, the geography of SPARC+ Tregs is unknown, as is their role in homeostasis and disease. Looking at the data of the paper of Shahneh et al, it is likely that SPARC+ Tregs also are of importance in other diseases, such as atherosclerosis, stroke, and cancer. This paper will certainly SPARC increased interest into the immunobiology of DVT and beyond.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal