In this issue of Blood, Yoshino et al use the genetic Trib1-ROSA26-Cre–knockout murine model and retroviral-mediated HoxA9 overexpression, together or not with Trib1 reexpression (Trib1 Hi and Trib1 null), to investigate the mechanistic basis for HoxA9-mediated acute myeloid leukemia (AML).1 They have deciphered the mechanism involved in HoxA9-associated AML, linking an Erg-specific superenhancer with the Trib1-C/EBPα axis. It has previously been shown that TRIB proteins, including TRIB1 and TRIB2, are AML oncoproteins capable of driving disease development. Degradation of the p42 isoform of C/EBPα (an important myeloid transcription factor) and increased MAPK/ERK signaling (a proliferation and survival indicator) are key molecular mechanisms for Trib oncogenic activity.2 Cooperation with HoxA9 leads to accelerated AML development,3,4 which is important because HoxA9 overexpression alone is unable to drive AML in vivo. The role that TRIB proteins may have across the wide heterogeneity of AML phenotypes is still underappreciated; however, given that deregulated Hox signaling is associated with ∼70% of all AMLs, it is important to understand the involvement of Trib oncogenic activities in HoxA9-mediated AML. It remained an open question how TRIB proteins cooperate with Hoxa9 at the mechanistic level.
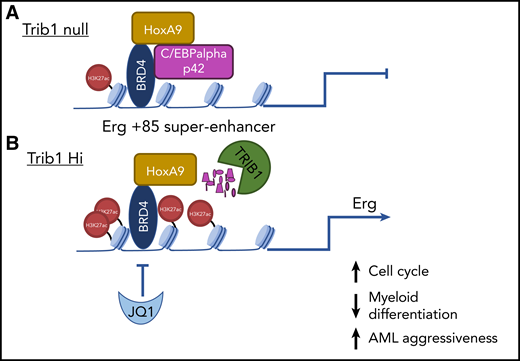
Schematic depiction of Erg +85 superenhancer in Trib1 null and Trib1 Hi HoxA9-expressing leukemia cells. (A) Trib1 null; (B) Trib1 Hi.
Schematic depiction of Erg +85 superenhancer in Trib1 null and Trib1 Hi HoxA9-expressing leukemia cells. (A) Trib1 null; (B) Trib1 Hi.
Previous data have shown that HoxA9 is organized into enhanceosomes containing lineage-restricted transcription factors, including C/EBPα.5 Together with what is known about TRIB1 oncoprotein function, this led the investigators to hypothesize that Trib1-mediated degradation of C/EBPα p42 isoform would lead to enhanced remodeling at HoxA9-associated genomic loci and, thus, be a key event in HoxA9-Trib1 cooperation in AML. The investigators tested this using microarray gene expression and chromatin immunoprecipitation sequencing analysis and could show that, in the absence of Trib1 (Trib1 null) in HoxA9-expressing cells, C/EBPα p42 is expressed, and a HoxA9-C/EBP complex and H3K27ac are present at distal enhancers. The HoxA9-C/EBP complex is associated with gene activation and repression and is known to contribute to the aberrant proliferative phenotype in an AML model that required coexpression of MEIS1.6 In this article, the investigators now advance our understanding of HoxA9 leukemogenesis (see figure); HoxA9 DNA binding peaks and H3K27ac peaks were not significantly different between TRIB1 hi and TRIB1 null cells, but HoxA9-associated superenhancers specific to TRIB1 hi AML cells were enriched, most notably at the Erg +85 superenhancer, together with H3K27ac. Importantly, it was the removal of C/EBPαa p42 isoform mediated by Trib1 that occurred at these specific HoxA9-associated superenhancer genomic loci leading to enhanced H3K27ac and elevated Erg expression. There is a requirement for C/EBPalpha expression in HoxA9-associated and TRIB-mediated AML,6,7 which can seem counterintuitive. However, this is explained in the literature by the expression and opposing functions of C/EBPα p42 and p30 isoforms. The degradation of p42 isoform, while p30 expression remains intact, is a feature of AML8 and is consistent with the literature; here, it is shown to be a driving force for enhancer modification at the HoxA9-binding specific Erg superenhancer.
The investigators tested the therapeutic targeting of this mechanism with the use of the BRD4 inhibitor JQ1, because BRD4 is abundant at superenhancers. JQ1 suppressed the leukemic superenhancer activity of TRIB1 hi AML cells in vitro and in vivo, consistent with a block in Erg target gene expression. It remains an open question to what degree the expressions of Hox proteins and Trib proteins are linked across AML. Using messenger RNA expression has not revealed strong correlations among HoxA9, Trib1 or Trib2, and Erg; however, as elegantly shown in this article, oncoprotein activity and protein isoform expression are the key factors distinguishing AML with highly aggressive features that can be therapeutically targeted. Erg expression is associated with poor prognosis in AML,9 as well as a more aggressive phenotype10 ; therefore, its elevated expression as a result of Trib1 function in HoxA9-associated leukemia has important implications for patient prognostication and, potentially, AML stratification.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal