In this issue of Blood, Bobillo et al1 present a retrospective analysis of outcomes in stage I diffuse large B-cell lymphoma (DLBCL) patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or R-CHOP–like regimens with or without radiation therapy (RT). While the overall outcome was excellent, patients with extranodal presentation had an inferior outcome compared with patients with nodal disease. Consolidation with RT improved progression-free survival (PFS) and overall survival (OS) in patients with extranodal disease, mainly due to improved outcomes in positron emission tomography (PET)–positive patients at the end of immunochemotherapy.
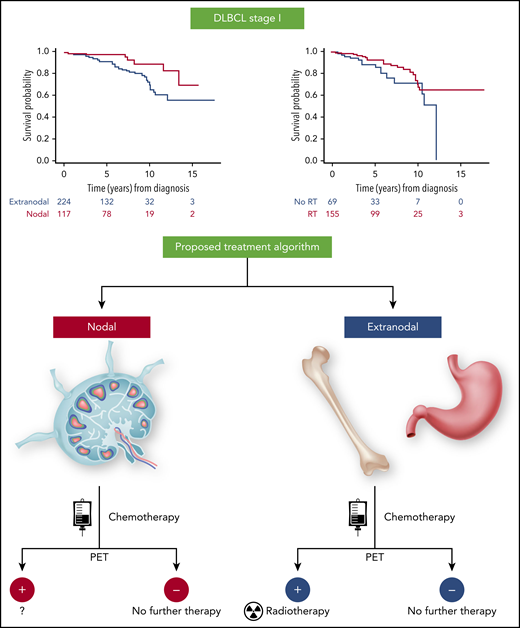
Bobillo et al demonstrated that in stage I DLBCL patients, OS and PFS were shorter in patients with extranodal disease than in patients with nodal involvement. Radiotherapy was associated with longer OS and PFS in patients with extranodal DLBCL. Based on these findings, a depicted treatment algorithm can be proposed. A small number of events did not allow analysis of the value of radiotherapy in patients with nodal presentation and positive PET after chemoimmunotherapy. The figure has been adapted from Figures 3A and 4 in the article by Bobillo et al that begins on page 39.
Bobillo et al demonstrated that in stage I DLBCL patients, OS and PFS were shorter in patients with extranodal disease than in patients with nodal involvement. Radiotherapy was associated with longer OS and PFS in patients with extranodal DLBCL. Based on these findings, a depicted treatment algorithm can be proposed. A small number of events did not allow analysis of the value of radiotherapy in patients with nodal presentation and positive PET after chemoimmunotherapy. The figure has been adapted from Figures 3A and 4 in the article by Bobillo et al that begins on page 39.
Limited-stage presentation accounts for ∼30% to 40% of DLBCL cases. Retrospective analyses and clinical trials in these patients demonstrated favorable but markedly variable outcomes following short- or full-course chemotherapy with or without rituximab with or without RT. Variability in outcomes in limited-stage DLBCL patients stems from different definitions of limited stage (stage I or both stage I and II), tumor bulk, and whether staging was performed by PET computed tomography. Differences in survival can also be attributed to the presence of unfavorable clinical characteristics that constitute the stage-modified International Prognostic Index (sm-IPI)2 (age >60 years, elevated lactate dehydrogenase, performance status ≥2, and stage II), bulky disease, and biological heterogeneity. While extranodal involvement, a risk factor in the original IPI, is not included in the sm-IPI, there are reports suggesting that extranodal presentation may be associated with inferior outcomes.3
Consequently, the optimal therapy for limited-stage DLBCL patients in general and risk-stratified patient subgroups remains unclear.
In the pre-rituximab era, 4 randomized trials in limited-stage DLBCL patients have compared chemotherapy with combined modality treatment2,4-6 but did not establish the best treatment approach. The SWOG study S8736 enrolled stage I (including bulky [>10 cm]) and nonbulky stage II patients without any restrictions based on the sm-IPI.2 The initial report showed an OS advantage with 3 cycles of CHOP combined with RT over 8 cycles of CHOP. However, with longer follow-up, this survival advantage disappeared due to continuous late relapses, which were also observed in other studies.
In the rituximab era, 2 randomized trials demonstrated that (1) RT may not be necessary in nonbulky (<7 cm) stage I/II patients achieving a PET-based complete response after 4 cycles of R-CHOP,7 and (2) in younger patients (≤60 years) with nonbulky (<7.5 cm) stage I/II disease without risk factors based on the age-adjusted IPI, 4 cycles of CHOP with 6 cycles of rituximab is noninferior to 6 cycles of R-CHOP.8 However, most patients enrolled in these trials had no risk factors for inferior survival, and they did not address the role of RT in high-risk patients. Furthermore, these studies did not include patients with bulky disease, and only 30% to 40% of patients had extranodal disease.
Bobillo et al report the results of a retrospective analysis of 354 stage I DLBCL patients (66% extranodal and 34% nodal) without any inclusion restrictions. Patients were treated with R-CHOP or R-CHOP-like regimens with or without RT. Treatment response was assessed by PET in 93% of patients. The 5-year disease-free survival and OS of all patients were 77% and 94%, respectively. Using a multinomial propensity score weighted analyses, the authors found that patients with extranodal disease had shorter OS and PFS than patients with nodal involvement. RT consolidation prolonged patient OS. In multivariate analyses, RT consolidation was associated with longer OS and PFS in the extranodal patients, but no such analysis could be performed in patients with nodal disease due to a small number of events. In a landmark analysis of patients alive at 6 months after diagnosis who had PET imaging at the end of chemotherapy prior to RT, a positive scan was not associated with shorter PFS in the whole cohort and in patients with extranodal disease. Further, the benefit of RT was no longer observed in patients with negative PET at the end of immunochemotherapy. Overall, these findings suggest that PET-positive patients with extranodal presentation may benefit from RT (see figure).
The authors should be commended for performing this study and addressing the prognostic significance of extranodal presentation in stage I DLBCL and demonstrating that these patients may benefit from RT, especially if PET after chemotherapy is positive. Currently, there is a tendency to forego RT in patients with limited-stage DLBCL based on the data in low-risk patients. However, RT may be beneficial in specific high-risk patients or following insufficient response to immunochemotherapy. In the UNFOLDER (unfavorable low-risk patients treated with densification of R-chemo regimens) trial, patients with an age-adjusted IPI of 0 with bulky (>7.5 cm) disease or IPI of 1 were randomly assigned to R-CHOP with or without RT to bulky and/or extranodal disease.9 The 3-year event-free survival (EFS) was superior in patients who received RT. The reduced EFS in patients without RT was linked to a higher rate of partial responses that triggered additional treatment. However, there was no difference in PFS and OS, indicating that additional studies addressing the role of RT in extranodal DLBCL may be needed.
There are several limitations to this retrospective study. The chemotherapy was not uniform, and patient treatments were selected by physicians based on clinical presentation and other unknown but potentially confounding considerations. The small number of patients in individual treatment groups prevented comparison of outcomes between patients treated with an abbreviated vs a full course of chemotherapy. The small number of patients with nodal presentation and PET positivity after chemotherapy did not allow an analysis of the role of RT in these patients. There was variability in time of PET performance after chemoimmunotherapy. The extranodal stage I patients in this analysis included patients with testicular and breast disease, which are associated with a peculiar clinical course (eg, contralateral and central nervous system relapses)10 ; based on compelling retrospective data, these patients should be treated differently than patients with extranodal disease. Moreover, no biopsies were performed in patients with PET-positive disease at the end of immunochemotherapy to differentiate between residual disease and inflammatory changes from treatment that may be unique to extranodal sites (eg, bone remodeling in osseous presentations). Finally, as with every retrospective study, we must be careful to not overinterpret the data but use it for hypothesis generation to be tested in a prospective randomized trial.
Conflict-of-interest disclosure: The author declares no competing financial interests.