Neoantigens are generated by mutations acquired in malignant cells. To be immunogenic, neoantigen peptides must be processed and then presented by HLA molecules on the tumor cell surface to evoke a T cell response. Emerging evidence suggests neoantigens are promising new targets of personalised cancer immunotherapies, provided that antigen presentation remains intact. Recently, we (Tobin, JCO 2019) demonstrated the link between the tumor microenvironment (TME) and clinical outcome in patients with advanced-stage Follicular Lymphoma (ASFL). In particular, ASFL patients with favorable outcomes had high levels of immune infiltration (best stratified by PD-L2 expression) with increased intratumoral CD8+ T cell clonotypes suggestive of expanded antigen-specific T cells in response to presentation by HLA class I neoantigens. This is supported by HLA class I pathway genetic aberrations being infrequent (unlike HLA class II) in FL. However, to date there is minimal data on the frequency and nature of putative neoantigens, their relationship with HLA class I antigen presentation and with the TME in any stage of FL. In particular, the immunobiology of early-stage FL (ESFL) has been largely neglected. Here, we present detailed characterization of these parameters in patients with early-stage FL (ESFL) from the TROG99.03 prospective clinical trial (MacManus, JCO 2018).
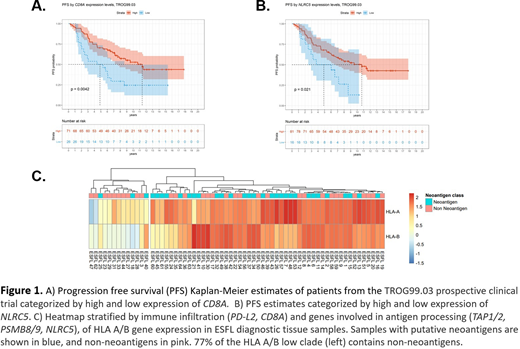
Digital multiplex gene expression (770 gene PanCancer Immune Panel, NanoString) and targeted NGS (365 genes recurrently mutated in hematological malignancies) was performed on 97 FFPE diagnostic tissue samples. Intratumoral CD8A expression cut-off derived by maximally selected rank statistics was associated with >2-fold differential median PFS (CD8Ahi: 11.5 years vs. CD8Alo: 4.9 years, p=0.0042, Figure 1A). In keeping with a relationship between HLA class I immune effector infiltration within the TME and FL tumor antigen presentation/processing, expression of NLRC5 (a transcriptional activator of HLA class I) was also associated with differential median PFS (NLRC5hi: 10.8 years vs. NLRC5lo: 4.9 years, p=0.021, Figure 1B). The impact of expression of CD8A and NLRC5 were independent of mutations in BCL-2,TP53 and all but of one the m7-FLIPI genes. The exception was EZH2, mutations of which were enriched in cases with reduced CD8A (p=0.0066) and NLRC5 (p<0.0001), broadly consistent with prior observations in DLBCL (Ennishi, Can Disc 2019).
Next, we investigated the expression of HLA class I and predicted neoantigens. HLA genotyping was performed using a custom sequencing panel on matched germline peripheral blood DNA for 71 TROG99.03 diagnostic samples. For the corresponding somatic coding variants, the strongest HLA class I binding efficiencies were calculated using 8 epitope prediction algorithms. 41% of samples had no detectable neoantigens, whereas 59% of samples had ≥1 neoantigens detected, with 116 neoantigens total. Importantly, unsupervised hierarchical clustering showed 2-fold enrichment of samples with neoantigens among those with elevated HLA A/B expression vs. samples with low HLA A/B (p=0.0023, Figure 1C).
Interestingly, roughly one third of neoantigens were due to indels causing frameshift events resulting in the generation of 'non-self' peptides with strong predicted binding affinity. Recently TCGA data has been used to show that frameshift variants occurring at different sites in a gene can converge to generate a handful neo open reading frame peptides ('NOPs') common to multiple cancer patients. Therefore, it has been postulated that these NOPs could have a considerable therapeutic potential as a novel pan-cancer 'semi-personalized' immunotherapy. In keeping with data derived in solid tumors, in ESFL we observed frameshift variants were most frequently observed in KMT2D.
Finally, 5 relapsed/transformed FL samples were compared to their paired diagnostic tissue samples. Not only did neoantigens present at diagnosis typically remain detectable, but in several cases additional neoantigens were also found.
In summary, we demonstrate for the first time in ESFL that neoantigens are typically associated with intact HLA class I presentation; CD8 infiltration within the TME is related to tumor antigen processing and associated with favorable outcomes; and that a subset of FL tumors have NOPs. These data have important implications for the design of innovative next-generation immunotherapies in FL.
Keane:BMS: Research Funding; Celgene: Honoraria, Other: Travel; Roche: Honoraria, Other: Travel, Speakers Bureau; MSD Oncology: Honoraria, Other: Travel; Gilead: Honoraria, Other: Travel, Speakers Bureau. Blombery:Amgen: Consultancy; Novartis: Consultancy; Janssen: Honoraria; Invivoscribe: Honoraria. Tobin:Gilead: Research Funding. Seymour:Nurix: Honoraria; Morphosys: Consultancy, Honoraria; Mei Pharma: Consultancy, Honoraria; Gilead: Consultancy; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Gandhi:Celgene: Research Funding; Bristol-Myers Squibb: Research Funding; Merck Sharp & Dohme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Gilead Sciences: Honoraria; Genentech: Honoraria; Mater Research: Current Employment; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Other: Travel, accommodation, expenses .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal