Background
With the emergence of immunotherapies as a promising cancer treatment, there is now a pressing need for pre-clinical animal models to test immunotherapy strategies for acute myeloid leukemia (AML). While murine xenotransplant models generated by transplanting human AML cells are frequently used to model AML, they require immune-deficient mice. Syngeneic murine bone marrow transplant leukemia models (MBMTLM), which are established in immune competent mice, usually require radiation of the recipient mouse before leukemic cells are transplanted. This radiation suppresses the immune system and makes it difficult to study how the immune system responds to leukemia cells.
Aim
Our aim was to establish immunocompetent MBMTLMs to understand how the immune system responds to leukemia cells expressing a highly immunogenic antigen.
Method
We first established MBMTLMs models driven by the CALM/AF10 or MLL/AF9 fusion genes. Then leukemia cells were transduced with a SIINFEKL expressing retrovirus (MSCV-DsRed-SIINFEKL). SIINFEKL is a highly immunogenic eight amino acid peptide from ovalbumin.
Results
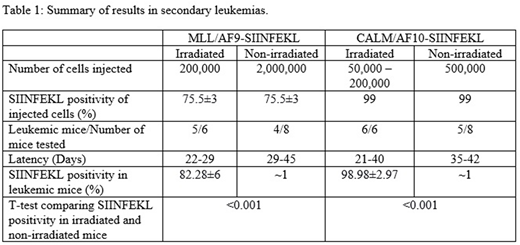
The transduction efficiency of MSCV-DsRed-SIINFEKL was about 12.9% on leukemic cells of MLL/AF9 and 13% on CALM/AF10 cells. All primary MLL/AF9-SIINFEKL (n=3) developed leukemia with a latency of 22 days. SIINFEKL expression was detected on 75.5±3% of the spleen cells of these mice. These spleen cells were transplanted into irradiated (n=6) and non-irradiated (n=8) mice to establish secondary MLL/AF9-SIINFEKL leukemias (Table 1). In the irradiated recipients, five out of six mice developed leukemia within 22-29 days. In non-irradiated recipients, four out of eight developed leukemia with a latency of 29-45 days. Flow cytometry showed that SIINFEKL was expressed on 82.28±6% of the spleen cells in irradiated recipients. In contrast, fewer than 1% of the spleen cells in non-irradiated mice with secondary MLL/AF9-SIINFEKL leukemia expressed SIINFEKL. Similar experiments were performed with the CALM/AF10 model. All primary CALM/AF10-SIINFEKL transplanted mice (n=2) developed leukemia with a latency of 32 and 42 days and showed SIINFEKL positivity on 99% of the leukemia cells. Secondary CALM/AF10-SIINFEKL leukemias were generated by transplanting these AML cells into irradiated (n=6) and non-irradiated (n=8) mice. All six irradiated recipients developed leukemia within 21-40 days. However, only five of the eight non-irradiated recipients developed leukemia with a latency of 35-42 days. Strikingly, about 99% of the AML cells in the irradiated mice were SIINFEKL positive but fewer than 1% of the AML cells in the five non-irradiated mice who developed leukemia were SIINFEKL positive.
Discussion
We find that for both MLL/AF9 and CALM/AF10 AML an intact immune system (non-irradiated recipients) is able to largely eliminate AML cells which express the SIINFEKL antigen, even when challenged with a large number of AML cells. In the non-irradiated recipients only 50 to 60% developed leukemia, and the leukemias that did develop had hardly any SIINFEKL-positive cells. In contrast, nearly all irradiated mice (11 of 12) developed leukemia with a high percentage of SIINFEKL expressing AML cells. SIINFEKL was presented on the surface of leukemic cells by the murine MHC class I H-2Kb molecule. Our results differ slightly from research reported by Hasegawa et al. 2015, who showed that MLL/AF9 AML cells expressing ovalbumin caused leukemia after transplantation into non-irradiated mice. However, they did not investigate whether ovalbumin was still being expressed on the leukemia cells.
Conclusion
We have established syngeneic murine AML models in immunocompetent mice and have evidence that an intact immune system has the ability to suppress or even eliminate rapidly proliferating AML cells if they express a strong antigen. These models should be useful for developing immunotherapy strategies for AML.
Desai:University of Auckland: Current Employment. Kakadia:University of Auckland: Current Employment. Browett:University of Auckland: Current Employment; BeiGene: Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Shire: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Bohlander:University of Auckland: Current Employment; Family of Marijana Kumerich: Research Funding; Leukaemia and Blood Cancer New Zealand: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal