Introduction Fanconi anemia (FA) is the most common inherited bone marrow failure syndrome with a surged cancer incidence, especially in hematologic malignancies, which has been listed in the entity of myeloid neoplasms with germline predisposition in the 2016 revision to the World Health Organization classification of tumors of hematopoietic and lymphoid tissues.Whether FA heterozygotes are predisposed to bone marrow failure and hematologic neoplasm is crucial but unsettled. We therefore retrospectively analyzed rare possibly significant variations (PSVs) in the five most obligated FA genes, BRCA2, FANCA, FANCC, FANCD2, and FANCG, in 788 aplastic anemia (AA) and hematologic malignancies patients to address this issue.
Methods Patients diagnosed as AA, myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), and acute lymphocytic leukemia (ALL) from April 2015 to December 2018 in Hebei Yanda Lu Daopei Hospital were enrolled with the ones diagnosed as FA via chromosome breakage test and/or genetic test excluded. Detailed disease histories and workup files were retrieved from the electronic medical record. Amplicon-based high throughput sequencing of aforementioned five genes were performed. The significance of the germline missense variants was assessed by in silico prediction algorithms, including SIFT, Polyphen2, PROVEAN, FATHMM, MutationTaster, and MutationAssessor. Variant predicted as deleterious/possibly deleterious by ≥ 3/6 scoring tools was defined as rare PSV and further included in statistical analysis. For splice site mutations, GeneSplicer, Human Splicing Finder, NetGene2, and FSPLICE were employed and only when ≥2/4 algorithms predict to affect/possibly affect splicing, would the variant be regarded as possibly significant. Same criteria were adopted when stratifying variants recorded in the ExAC. All reported variants in this study were confirmed germline variants by Sanger sequencing with fingernail specimens and/or pedigree analysis.
WFisher exact two-tailed test was adopted for variant frequency comparison. Development of disease was analyzed by cumulative incidence method and Kaplan-Meier method. p < 0.05 was considered as statistically significant.
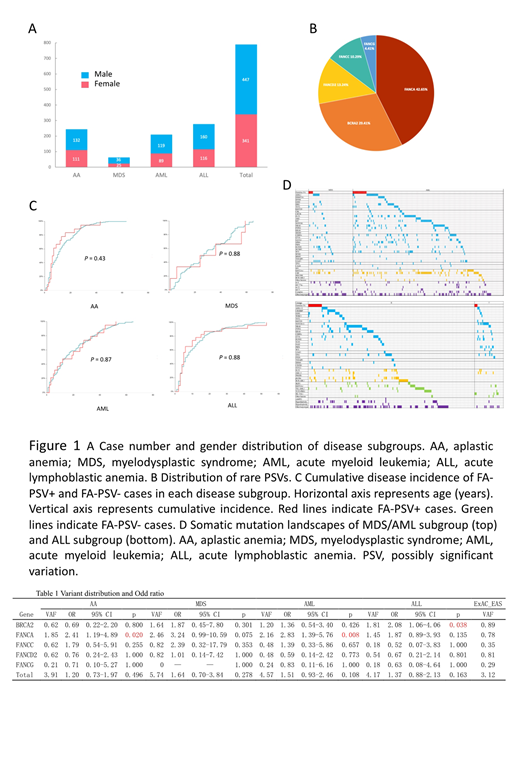
Results A total of 788 patients, who were all of the East Asian ancestry, were enrolled, including 341 females and 447 males (Figure 1A). The median age of onset was 10 (1-63), 27 (1-65), 14.5 (1-65), and 6 (1-53) years in AA, MDS, AML, and ALL subgroup, respectively. Sixty-eight variants were identified in 66 patients (8.38%). FANCA is the most frequently mutated gene (n = 29), followed by BRCA2 (n = 20) (Figure 1B). When compared to the ExAC East Asian dataset, there was an overall higher rare PSVs incidence in our cohort (p = 0.016). BRCA2 PSVs showed a higher frequency in ALL (p = 0.038), and FANCA PSVs were significantly enriched in AA and AML subgroups (p = 0.020; p = 0.008). The patients with FANCA heterozygotes also tended to show an increased risk for developing MDS (p = 0.075) (Table 1). No impact of FA-PSV status was found neither on cumulative disease incidence (Figure 1C). FA-PSV + MDS/AML patients have a heavier tumor mutation burden, higher rate of cytogenetic abnormalities, and less epigenetic regulation and spliceosome gene mutations than those of FA-PSV - MDS/AML patients (p = 0.024, p = 0.029, p = 0.024, and p = 0.013) (Figure 1D).
Discussion The overall PSVs enrichment in our cohort buttresses that heterozygous mutations of FA genes abate the capacity of DNA homologous recombination repair pathway and contribute to hematopoietic failure. Furthermore, we present the first evidence that BRCA2 heterozygotes have a significantly higher risk of developing into ALL. Instead of all or nothing, the impact of different variant imposed on protein might be seen as a continuous variation. Therefore, contributions of the FA pathway defect could be latent and subtle but be profound as time goes by. We further firstly observed a higher incidence of cytogenetic abnormalities and somatic mutations with statistical significance, and lower frequency of epigenetic regulation and spliceosome gene mutations in FA-PSV + myeloid malignancies. This provides evidence that these FA-PSV carriers are prone to accumulate chromosomal structural abnormalities, and confer the congenital susceptibility of myeloid malignancies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal