Introduction: Treatment of older or frail patients (pts) with multiple myeloma (MM) remains challenging due to impaired organ function, underlying comorbidities, need for convenient regimens and burden of care, all of which impact the tolerability of anti-MM therapies. In the STORM study, selinexor plus dexamethasone (sel-dex) demonstrated anti-MM activity with an ORR of 26.2% and similar safety across all age groups in pts with triple-class refractory MM (Chari et al. NEJM 2019, Gavriatopoulou et al. IMW 2019). In the phase 3 BOSTON study, the combination of weekly sel-dex with once weekly bortezomib (SVd) was superior to standard twice weekly bortezomib and dex (Vd) across all efficacy endpoints in pts with MM who had received 1-3 prior therapies. Furthermore, while conferring a longer PFS and higher ORR than Vd, SVd was associated with significantly reduced rates of peripheral neuropathy (PN), the most clinically relevant toxicity associated with bortezomib that limits long term administration, especially in older and/or frail pts. Here we present results of subgroup analyses of the BOSTON study to evaluate the safety and efficacy of SVd versus Vd based on age and frailty index.
Methods: Pts enrolled in BOSTON were assigned (1:1) to once weekly oral sel (100 mg) plus once weekly subcutaneous (SC) bortezomib (1.3 mg/m2) and dex (20 mg BIW) in the SVd arm or to standard twice weekly SC bortezomib (1.3 mg/m2) and dex (20 mg QIW) in the Vd arm. Treatment was administered in both arms until disease progression. The primary endpoint was PFS, as assessed by an Independent Review Committee. For these analyses, pts were assigned to 2 groups based on age (<65 and ≥65 years) and Frailty Score categories (frail and fit). Frailty Score was assessed using baseline characteristics including age, Charlson Comorbidity Index, and Eastern Cooperative Oncology Group performance status (Facon et al. Leukemia 2019).
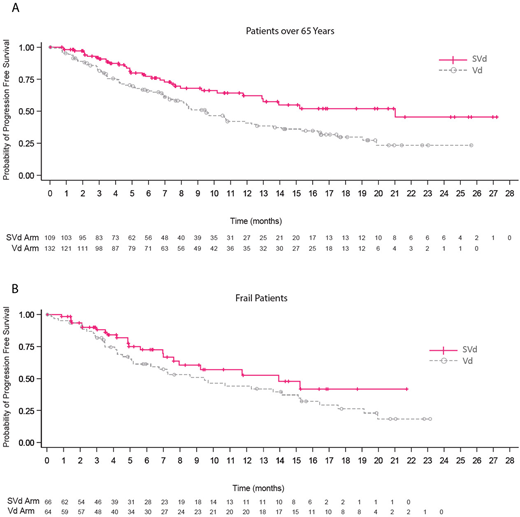
Results: Of the 402 enrolled pts, 241 (60%) were ≥65 years (SVd=109, Vd=132). Of these, 81 (37%) were ≥75 and 106 (44%) were categorized as frail (SVd=51, Vd=55). Baseline pt and disease characteristics were well balanced between treatment arms in the subgroups. PFS was prolonged in both age groups with SVd compared with Vd. In ≥65, PFS was 21.0 vs 9.5 months (HR, 0.55; 95% CI, 0.37-0.83; P=0.0018) and in <65, PFS was 12.2 vs 9.4 months (HR, 0.74; 95% CI, 0.41-1.10; P=0.07). The PFS benefit of SVd was sustained and comparable in the frail pts: 13.9 vs 9.5 months (HR, 0.69; 95% CI, 0.40-1.17; P=0.08); and in the fit pts: 13.2 vs 9.4 months (HR, 0.66; 95% CI, 0.47-0.93; P=0.0076) (Figure). The ORR was significantly improved with SVd in those ≥65 (76.1% vs 64.4%; P=0.0243) and <65 (76.7% vs 58.7%, P=0.0071). The ORR was improved with SVd in both the frail (69.7% vs 60.9%; P=0.14) and fit groups (79.8% vs 62.9%; P=0.0011). Overall survival was 24.8 months and 23.5 months in the Vd arm in the ≥65 and frail pts respectively and was not reached in any of the subgroups in the SVd arm. There were more deaths in the ≥65 (30% [SVd 23%, Vd 36%]) and frail groups (35% [SVd 26%, Vd 44%]) compared with the <65 (23% [SVd 26%, Vd 20%]) and fit groups (24% [SVd 23%, Vd 24%]). Similar to the overall population, the most common grade ≥3 adverse events (AEs) were thrombocytopenia, anemia, pneumonia and fatigue. In the SVd arm, the incidence of AEs was comparable across subgroups except for a higher incidence of fatigue in ≥65 versus <65 (17% vs 8%) and pneumonia in the frail versus fit (19% vs 7%). The incidence of serious AEs in the SVd versus Vd arms was (56% vs 45%) and (47% vs 25%) in ≥65 and <65 and (59% vs 48%) and (48% and 33%) in the frail and fit groups, respectively. Treatment discontinuation due to AEs occurred in 21% pts on SVd versus 16% on Vd in ≥65 and in 18% on SVd versus 11% on Vd in the frail group. Grade ≥ 2 PN rates were lower in the SVd compared with Vd arms in all subgroups with significant differences in ≥65 (22% vs 37%; P=0.0060) and frail groups (15% vs 44%; P=0.0002).
Conclusions: In pts with previously treated MM, the once weekly SVd regimen led to prolonged PFS, improved response rates and rates of PN regardless of age and frailty score compared to standard twice weekly Vd. Non-PN AEs were higher with triplet than doublet therapy, but most of the AEs were reversible and treatable. There were fewer deaths on SVd in pts ≥65 and in frail pts compared to Vd. Once weekly SVd is a potent and convenient treatment option for pts with previously treated MM, including those who are ≥65 years and/or frail.
Auner:Karyopharm: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding. Gavriatopoulou:Amgen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Genesis Pharma: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Delimpasi:GENESIS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Spicka:Celgene, Amgen, Janssen-Cilag, Takeda, Bristol-Myers Squibb, Novartis, Sanofi: Consultancy, Honoraria, Speakers Bureau. Dimopoulos:BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau. Leleu:Incyte: Honoraria; Merck: Honoraria; AbbVie: Honoraria; Carsgen: Honoraria; Janssen: Honoraria; Novartis: Honoraria; BMS-celgene: Honoraria; Amgen: Honoraria; GSK: Honoraria; Sanofi: Honoraria; Karyopharm: Honoraria; Oncopeptide: Honoraria. Hajek:Celgene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Pharma MAR: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy, Honoraria, Research Funding. Sinha:Dr Reddys Lab, Intas Pharmaceuticals, Karyopharm Therapeutics: Honoraria. Venner:Janssen, BMS/Celgene, Sanofi, Takeda, Amgen: Honoraria; Celgene, Amgen: Research Funding. Garg:Janssen, Takeda, Celgene, Novartis, Sanofi: Honoraria. Stevens:Amgen, MorphoSys: Consultancy. Quach:Amgen, Celgene, karyopharm, GSK, Janssen Cilag, Sanofi.: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline, Karyopharm, Amgen, Celgene, Janssen Cilag: Honoraria; Amgen, sanofi, celgene, Karyopharm, GSK: Research Funding; GlaxoSmithKline, Karyopharm, Amgen, Celgene, Janssen Cilag: Consultancy. Jagannath:Sanofi: Consultancy, Honoraria; Legend Biotech: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Moreau:Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; Novartis: Honoraria. Levy:Takeda: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Bristol Meyers Squibb: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Research Funding, Speakers Bureau; Baylor University Med Center: Current Employment. Badros:Amgen: Consultancy; University of Maryland: Current Employment. Anderson:BMS: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Bahlis:BMS/Celgene and Janssen: Consultancy, Honoraria, Other: Travel, Accomodations, Research Funding; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Karyopharm Therapeutics: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Facon:Celgene, Janssen, Takeda, Amgen, Roche, Karyopharm, Oncopeptides, BMS, Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Mateos:EDO Mundipharma: Honoraria; Seattle Genetics: Honoraria; Abbvie: Honoraria; Janssen: Honoraria; Bristol-Myers Squibb: Honoraria; Amgen: Honoraria; GlaxoSmithKline: Honoraria; Adaptive Biotechnologies: Honoraria; Takeda: Honoraria; Roche: Honoraria. Cavo:Jannsen, BMS, Celgene, Sanofi, GlaxoSmithKline, Takeda, Amgen, Oncopeptides, AbbVie, Karyopharm, Adaptive: Consultancy, Honoraria. Joshi:Karyopharm Therapeutics Inc: Consultancy. Chai:Karyopharm Therapeutics Inc: Current Employment. Arazy:Karyopharm Therapeutics Inc.: Current Employment. Shah:Karyopharm Therapeutics Inc: Current Employment, Current equity holder in publicly-traded company. Shacham:Karyopharm: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: (8999996, 9079865, 9714226, PCT/US12/048319, and I574957) on hydrazide containing nuclear transport modulators and uses, and pending patents PCT/US12/048319, 499/2012, PI20102724, and 2012000928) . Kauffman:Karyopharm Therapeutics Inc: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees. Richardson:Celgene/BMS, Oncopeptides, Takeda, Karyopharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal