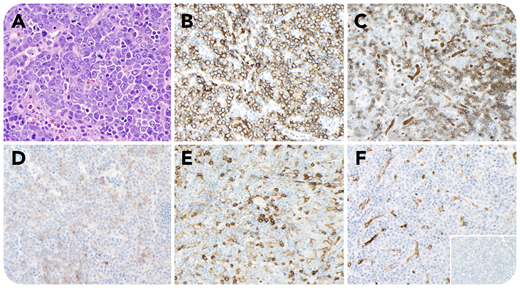
A 32-year-old man with anemia (hemoglobin, 9.9 g/dL) and thrombocytopenia (platelets, 76 × 109/L) had a bone marrow biopsy showing a myeloid neoplasm with PCM1-JAK2 fusion with left-shifted hyperplasia without increase in blasts, mild megakaryocytic atypia, and marked reticulin fibrosis. A computed tomography scan showed extensive lymphadenopathy and splenomegaly. An axillary lymph node biopsy was performed showing partial effacement by a diffuse infiltrate of large atypical cells with moderate basophilic cytoplasm, round nuclear contours, vesicular chromatin, prominent nucleoli, and evidence of sinusoidal involvement (panel A; hematoxylin and eosin stain, 40× objective, original magnification ×400). These cells were notably positive for E-cadherin (panel B; 20× objective, original magnification ×200), glycophorin A (subset, panel C; 20× objective, original magnification ×200), CD117 (subset, panel D; 20× objective, original magnification ×200), and EMA, and negative for CD45 (panel E; 20× objective, original magnification ×200), CD34 (panel F; 20× objective, original magnification ×200), MPO (panel F inset; 20× objective, original magnification ×200), CD3, CD20, CD30, and TdT, supporting a diagnosis of myeloid sarcoma with erythroblastic differentiation. The diagnosis was subsequently confirmed by fluorescence in situ hybridization testing, which was positive for evidence of a rearrangement involving JAK2.
This represents a unique case of tissue involvement by the patient’s known myeloid neoplasm with transformation to erythroblastic sarcoma. Although such myeloid neoplasms have been reported to transform into acute myeloid or lymphoblastic leukemia, transformation into erythroblastic sarcoma has not been previously found.
A 32-year-old man with anemia (hemoglobin, 9.9 g/dL) and thrombocytopenia (platelets, 76 × 109/L) had a bone marrow biopsy showing a myeloid neoplasm with PCM1-JAK2 fusion with left-shifted hyperplasia without increase in blasts, mild megakaryocytic atypia, and marked reticulin fibrosis. A computed tomography scan showed extensive lymphadenopathy and splenomegaly. An axillary lymph node biopsy was performed showing partial effacement by a diffuse infiltrate of large atypical cells with moderate basophilic cytoplasm, round nuclear contours, vesicular chromatin, prominent nucleoli, and evidence of sinusoidal involvement (panel A; hematoxylin and eosin stain, 40× objective, original magnification ×400). These cells were notably positive for E-cadherin (panel B; 20× objective, original magnification ×200), glycophorin A (subset, panel C; 20× objective, original magnification ×200), CD117 (subset, panel D; 20× objective, original magnification ×200), and EMA, and negative for CD45 (panel E; 20× objective, original magnification ×200), CD34 (panel F; 20× objective, original magnification ×200), MPO (panel F inset; 20× objective, original magnification ×200), CD3, CD20, CD30, and TdT, supporting a diagnosis of myeloid sarcoma with erythroblastic differentiation. The diagnosis was subsequently confirmed by fluorescence in situ hybridization testing, which was positive for evidence of a rearrangement involving JAK2.
This represents a unique case of tissue involvement by the patient’s known myeloid neoplasm with transformation to erythroblastic sarcoma. Although such myeloid neoplasms have been reported to transform into acute myeloid or lymphoblastic leukemia, transformation into erythroblastic sarcoma has not been previously found.
For additional images, visit the ASH Image Bank, a reference and teaching tool that is continually updated with new atlas and case study images. For more information, visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal