Key Points
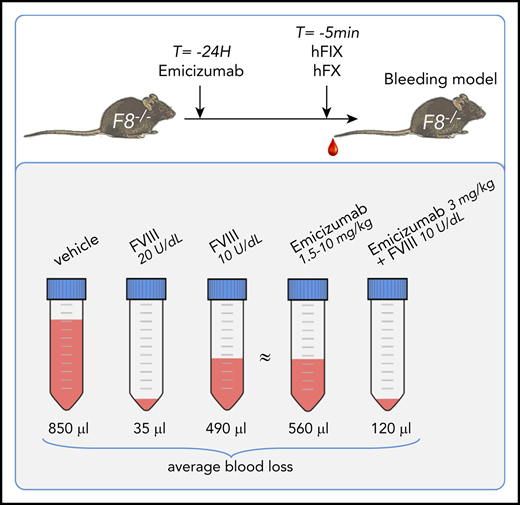
Emicizumab (≥1.5 mg/kg) partially corrects blood loss in a hemophilia A bleeding model, with an FVIII equivalence of 9 U/dL.
Emicizumab provides additive hemostatic activity when given in combination with low-dose FVIII.
Abstract
The bispecific antibody emicizumab is increasingly used for hemophilia A treatment. However, its specificity for human factors IX and X (FIX and FX) has limited its in vivo functional analysis to primate models of acquired hemophilia. Here, we describe a novel mouse model that allows emicizumab function to be examined. Briefly, FVIII-deficient mice received IV emicizumab 24 hours before tail-clip bleeding was performed. A second infusion with human FIX and FX, administered 5 minutes before bleeding, generated consistent levels of emicizumab (0.7-19 mg/dL for 0.5-10 mg/kg doses) and of both FIX and FX (85 and 101 U/dL, respectively, after dosing at 100 U/kg). Plasma from these mice display FVIII-like activity in assays (diluted activated partial thromboplastin time and thrombin generation), similar to human samples containing emicizumab. Emicizumab doses of 1.5 mg/kg and higher significantly reduced blood loss in a tail-clip–bleeding model using FVIII-deficient mice. However, reduction was incomplete compared with mice treated with human FVIII concentrate, and no difference in efficacy between doses was observed. From this model, we deducted FVIII-like activity from emicizumab that corresponded to a dose of 4.5 U of FVIII per kilogram (ie, 9.0 U/dL). Interestingly, combined with a low FVIII dose (5 U/kg), emicizumab provided enough additive activity to allow complete bleeding arrest. This model could be useful for further in vivo analysis of emicizumab.
Introduction
Limitations hampering factor VIII (FVIII)-replacement therapy in hemophilia A have spurred the development of novel therapeutic approaches, including the bispecific antibody emicizumab.1,2 Emicizumab bridges the enzyme FIXa and the substrate FX, thereby stimulating FX activation,3-5 and markedly reduces bleeding tendency in hemophilia A patients, with and without inhibitors.6-9 Unfortunately, current assays that measure emicizumab’s FVIII-like activity are unfit to predict an unbiased equivalence to true FVIII activity.5,10,11 Furthermore, in vitro assays allow only limited insight into the interaction between emicizumab and other procoagulant drugs.12,13
Because emicizumab selectively recognizes human and primate FIX and FX, reports that explored emicizumab in experimental models are scarce. We have therefore developed a first murine model that allows for a direct comparison between emicizumab and FVIII in vivo. In this model, emicizumab’s cofactor activity mimics FVIII levels of 9 U/dL. Additionally, emicizumab provides additional procoagulant activity at low concentrations of FVIII, resulting in enhanced hemostasis in FVIII-deficient mice.
Materials and methods
Animals and ethics statement
F8-deficient mice14 were backcrossed (>10 times) on a C57Bl/6 background. Male and female mice were used throughout the study (8-12 weeks old, 20-25 g). Housing and experiments were performed in accordance with French regulations and the experimental guidelines of the European Community. This project was approved by the local ethical committee of Université Paris-Sud (Comité d’Éthique en Experimentation Animale no. 26, protocol APAFIS#4400-2016021716431023v5).
Blood collection and plasma preparation
Blood was collected from ketamine/xylazine-anesthetized animals (100 mg/kg and 10 mg/kg, respectively) by retro-orbital puncture with a glass capillary in one-tenth volume trisodium citrate (13.8 mM). Plasma was prepared by centrifugation for 20 minutes at 1500g and stored at −80°C.
Proteins
Emicizumab (marketed under Hemlibra) was obtained from F. Hoffman-La Roche (Basel, Switzerland). Recombinant FIX (Benefix) was obtained from Pfizer Inc (Paris, France). Recombinant murine FIX was obtained from Sino Biologicals (Wayne, PA). Plasma-derived FIX and FX were obtained from Cryopep (Montpellier, France). Recombinant FVIII (Advate) was obtained from Shire France SAS (Paris, France). Polyclonal rabbit anti-FIX antibodies and polyclonal rabbit anti-human FX antibodies were isolated from serum obtained from rabbits immunized with human FIX or FX, respectively (Eurogentec, Seraing, Belgium). Purified antibodies were labeled with peroxidase using the EZ-link activated peroxidase antibody labeling kit as instructed (Thermo Fisher Scientific, Illkirch, France).
Antigen and activity assays
Human FIX antigen levels in murine plasma were determined in a FIX enzyme-linked immunosorbent assay (ELISA) using commercially available polyclonal sheep anti-human FIX antibodies (5 μg/mL; Cryopep) as coating antibody and peroxidase-labeled polyclonal rabbit anti-human FIX antibodies (diluted 1/1000) as probing antibodies. Human FX antigen levels in murine plasma were determined in an FX ELISA using commercially available polyclonal goat anti-human FX antibodies (5 μg/mL; Cedarlane, Gateshead, United Kingdom) as coating antibody and peroxidase-labeled polyclonal rabbit anti-human FX antibodies (diluted 1/3000) as probing antibodies. For both ELISAs, murine plasma, spiked with purified human FIX and FX, respectively, were used as reference. The limit of detection was 0.32 U/dL and 0.16 U/dL for the FIX and FX ELISAs, respectively.
FVIII activity was measured in a chromogenic assay using the Biophen FVIII-assay kit (Hyphen, Neuville-sur-Oise, France) as instructed.
Determination of emicizumab levels after infusion
Isoflurane-anesthetized mice were given emicizumab (0-10 mg/kg; 100-μL volume; n = 6 mice per dose) via IV injection in the retro-orbital venous plexus. Twenty-four hours after infusion, blood samples were taken under anesthesia via retro-orbital puncture from the opposite eye from where the protein was infused. Plasma was prepared and analyzed for the presence of human immunoglobulin G (IgG) using an immunosorbent assay. Briefly, diluted plasma samples were incubated in wells coated with a monoclonal anti-human IgG Fc antibody (10 μg/mL; Clinisciences, Nanterre, France). Bound emicizumab was probed using a peroxidase-labeled anti-human IgG4 Fc antibody (diluted 1/20 000; Abcam, Paris, France) and detected via peroxidase-mediated hydrolysis of 3,3′,5,5′-tetramethylbenzidine. Murine plasma, spiked with known concentrations of emicizumab, was used as reference. The limit of detection was 0.02 μg/mL.
Emicizumab-compatible mouse model
Isoflurane-anesthetized FVIII-deficient mice were given emicizumab (0-10 mg/kg; 100-μL volume; n = 6-18 per dose) via IV injection in the retro-orbital venous plexus 24 hours before the tail amputation. A second retro-orbital injection was given 5 minutes before the tail-amputation procedure to mice anesthetized with ketamine (100 mg/kg)/xylazine (10 mg/kg), using a solution containing human FIX and FX (both 100 U/kg, unless indicated otherwise). Subsequently, 3 mm of the distal tip of the tail was amputated. The amputated tail was immersed in prewarmed physiological saline (37°C) and blood was collected for 30 minutes. The mixture of collected blood and physiological saline was centrifuged at 1500g. The red blood cell pellet was then lysed in H2O and the amount of hemoglobin was determined by reading the absorbance at 416 nm. The volume of blood loss in each sample was calculated from a standard curve, which was obtained by lysing defined volumes of mouse blood (0.3-300 μL) in H2O to extract hemoglobin. The limit of detection is 0.6 μL of murine blood.
In some control experiments, mice were given emicizumab with FIX alone or FX alone. For some mice (n = 3-5), blood samples were taken after infusion of the emicizumab/FIX/FX cocktail (with emicizumab given 24 hours and FIX/FX given 5 minutes before blood collection), while these mice were not exposed to the tail-clip assay. These samples were used to measure FIX and FX antigen levels, and to test hemostatic activity in the activated partial thromboplastin time (aPTT) and FXIa-induced thrombin-generation assays.
FVIII-mediated correction of bleeding
Mice were given FVIII (0-10 U/kg; 100-μL volume; n = 9-18 per dose) either in the absence or presence of human FIX and FX (both 100 U/kg) via IV infusion in the retro-orbital venous plexus. The tail-clip–bleeding assay was performed 5 minutes after infusion as described in the previous section for the emicizumab-compatible mouse model. FVIII activity levels using the 2.5 to 10 tU/kg dosing varied dose-dependently between 5 and 20 U/dL.
Diluted aPTT
One-stage clotting activity was determined using the STA-PTTa-assay kit (Stago, Asnières, France). Briefly, plasma samples were diluted fourfold in STA-Owren-Koller buffer (Stago) to a volume of 50 μL. Subsequently, 25 μL of aPTT reagent were added. Finally, the clotting reaction was started by the addition of a 50-μL CaCl2 solution. Analysis was performed on a Diagnostica Stago ST4 Coagulation Analyzer.
FXIa-induced thrombin-generation assay
Thrombin generation in platelet-poor murine plasma was measured in a microtiter-plate fluorometer (Fluoroskan Ascent; Thermo Labsystems, Helsinki, Finland) according to the method described by Hemker et al, except that thrombin generation was initiated using phospholipids (4 μM) and FXIa (175 nM), respectively.15 Endogenous thrombin potential (ETP; ie, area under the curve) and thrombin peak as readouts for thrombin generation were determined using dedicated software (Thrombinoscope BV, Maastricht, The Netherlands). Plasma samples from the mice that received the emicizumab/FIX/FX or FVIII/FIX/FX cocktail, but did not undergo tail-clip bleeding, were used for this assay.
Statistical analysis
All data are presented as mean plus or minus standard deviation (SD) unless indicated otherwise. Number (n) refers to the number of independent experiments or animals. The statistical analysis was performed using GraphPad Prism 7 software for Mac (La Jolla, CA). One-way analysis of variance (ANOVA) followed by the Tukey or the Dunnett multiple comparison test was performed when comparing multiple groups. P < .05 was considered as statistically significant.
Results
An emicizumab-responsive mouse model
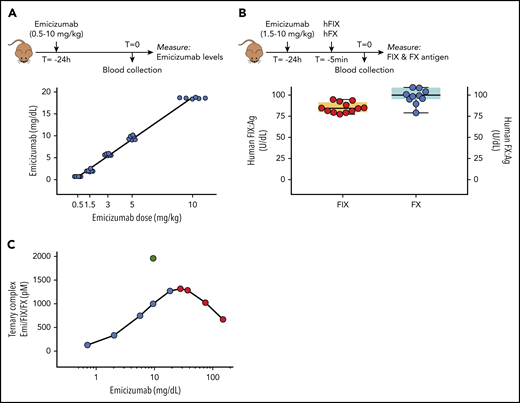
To test emicizumab in murine models, the presence of human FIX and FX is obligatory. Our approach therefore was to first administer emicizumab (0.5-10 mg/kg) via IV infusion into FVIII-deficient mice 24 hours before the tail-clip procedure. Subsequently, mice received a combined dose of human FIX and FX (both 100 U/kg) 5 minutes before tail amputation. Emicizumab levels 24 hours after infusion varied dose-dependently between 0.7 ± 0.1 mg/dL for the 0.5 mg/kg dose and 18.6 ± 14.1 mg/dL for the 10 mg/kg dose (Figure 1A). Dosing with 3 mg/kg generated plasma concentrations of 5.7 ± 0.2 mg/dL, similar to concentrations in patients who receive weekly subcutaneous dosing of 1.5 mg/kg.6 Human FIX and FX concentrations were measured in mice that received between 1.5 and 10 mg/kg emicizumab (n = 3 per dose), and pooled data revealed FIX and FX levels of 85 ± 6 U/dL (n = 12) and 100 ± 11 U/dL (n = 12; Figure 1B), respectively, at the time of tail amputation. No differences in FIX and FX levels between emicizumab doses were observed. Circulating ternary emicizumab/FIX/FX complexes were calculated to range between 129 pM and 1270 pM, based on the actual emicizumab and human FIX and FX levels at the time of the intervention (Figure 1C).
An emicizumab-responsive mouse model. (A) Mice received emicizumab (0-10 mg/kg) via retro-orbital infusion. Plasma samples obtained 24 hours after infusion were used to determine circulating emicizumab levels, expressed as milligrams per deciliter. Each data point represents an individual mouse. (B) FVIII-deficient mice previously infused with emicizumab (1.5-10 mg/kg), received a cocktail containing human FIX (hFIX) and FX (both 100 U/kg) via retro-orbital infusion. Plasma samples obtained 5 minutes after infusion were used to determine circulating levels of human FIX (left y-axis) and FX (right y-axis) antigen (Ag), expressed as units per deciliter. Each data point represents an individual mouse, and pooled data from the various emicizumab doses are shown. (C) Based on the reported affinity of emicizumab (Emi) for FIX (1.85 μM) and FX (1.58 μM) and the respective concentrations of emicizumab (0.7-18.6 mg/dL), FIX (85 U/dL), and FX (100 U/dL) in our model (see panels A-B), the concentration of the ternary emicizumab/FIX/FX complex for each emicizumab dose used in this study (blue symbols) was calculated as described.4 Higher doses of emicizumab will not further increase ternary complex formation, and may even lead to reduced ternary complex formation (red symbols). In contrast, increasing FIX and/or FX concentrations would allow for higher concentrations of ternary complexes (eg, green symbol: 9.6 mg/dL emicizumab, 200 U/kg FIX, 100 U/kg FX)
An emicizumab-responsive mouse model. (A) Mice received emicizumab (0-10 mg/kg) via retro-orbital infusion. Plasma samples obtained 24 hours after infusion were used to determine circulating emicizumab levels, expressed as milligrams per deciliter. Each data point represents an individual mouse. (B) FVIII-deficient mice previously infused with emicizumab (1.5-10 mg/kg), received a cocktail containing human FIX (hFIX) and FX (both 100 U/kg) via retro-orbital infusion. Plasma samples obtained 5 minutes after infusion were used to determine circulating levels of human FIX (left y-axis) and FX (right y-axis) antigen (Ag), expressed as units per deciliter. Each data point represents an individual mouse, and pooled data from the various emicizumab doses are shown. (C) Based on the reported affinity of emicizumab (Emi) for FIX (1.85 μM) and FX (1.58 μM) and the respective concentrations of emicizumab (0.7-18.6 mg/dL), FIX (85 U/dL), and FX (100 U/dL) in our model (see panels A-B), the concentration of the ternary emicizumab/FIX/FX complex for each emicizumab dose used in this study (blue symbols) was calculated as described.4 Higher doses of emicizumab will not further increase ternary complex formation, and may even lead to reduced ternary complex formation (red symbols). In contrast, increasing FIX and/or FX concentrations would allow for higher concentrations of ternary complexes (eg, green symbol: 9.6 mg/dL emicizumab, 200 U/kg FIX, 100 U/kg FX)
Ex vivo analysis of emicizumab-treated FVIII-deficient mice
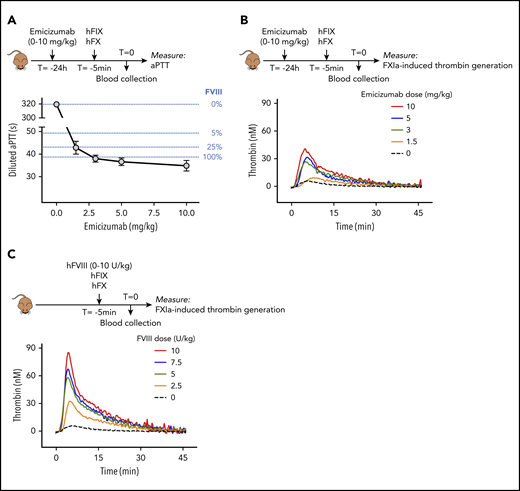
To investigate potential emicizumab cofactor activity under these conditions, murine plasma samples were analyzed for FVIII-like activity in a diluted aPTT assay and in an FXIa-dependent thrombin-generation assay. Diluted aPTT-clotting times were shortened dose-dependently and clotting times in the normal range were obtained at doses of 3 mg/kg or more (Figure 2A). Apparently, emicizumab fully corrects for FVIII deficiency in the diluted aPTT, akin to what was previously reported.10,11,16 This activity in this clotting assay is generally considered as an overestimation of the true FVIII equivalence of emicizumab.10,11,16 As for the FXIa-dependent thrombin-generation assay, emicizumab also promoted thrombin generation in this assay (Figure 2B). Maximal ETP was 511 ± 82 nM/min at 10 mg of emicizumab per kilogram, similar to that observed for mice that received 5 U of FVIII per kilogram (622 ± 107 nM/min; P = .149; Figure 2B-C; Table 1). In contrast, peak thrombin levels were significantly lower for emicizumab (42.2 ± 5.1 nM for 10 mg of emicizumab per kilogram and 58.3 ± 9.3 nM for 5 U of FVIII per kilogram, respectively; P = .0172; Figure 2B-C; Table 1). Together, these data highlight that, in an ex vivo setting, laboratory assays also give very different FVIII-equivalent values for emicizumab, similar to what has been observed in in vitro analyses.10 Nevertheless, coinfusing emicizumab with human FIX/FX allows for emicizumab-enhanced hemostasis in this mouse model.
Ex vivo analysis of emicizumab activity in murine plasma samples. (A) Plasma samples obtained from FVIII-deficient mice receiving emicizumab combined with human FIX/FX were analyzed for procoagulant activity in a diluted aPTT assay. Clotting times for each emicizumab dose are presented. Data represent mean plus or minus SD of 3 to 9 samples. Clotting times of murine FVIII-deficient plasma, spiked with known FVIII concentrations, are indicated as dotted blue lines for comparison. (B-C) Plasma samples obtained from FVIII-deficient mice receiving emicizumab combined with human FIX/FX (B) or FVIII combined with human FIX/FX (C) were analyzed in a FXIa-induced thrombin-generation assay. Data represent mean of 3 to 5 mice. Thrombin generation in the absence of emicizumab or FVIII (black dotted line) is the same for both panels. Data on lag time, ETP, and thrombin peak are presented in Table 1.
Ex vivo analysis of emicizumab activity in murine plasma samples. (A) Plasma samples obtained from FVIII-deficient mice receiving emicizumab combined with human FIX/FX were analyzed for procoagulant activity in a diluted aPTT assay. Clotting times for each emicizumab dose are presented. Data represent mean plus or minus SD of 3 to 9 samples. Clotting times of murine FVIII-deficient plasma, spiked with known FVIII concentrations, are indicated as dotted blue lines for comparison. (B-C) Plasma samples obtained from FVIII-deficient mice receiving emicizumab combined with human FIX/FX (B) or FVIII combined with human FIX/FX (C) were analyzed in a FXIa-induced thrombin-generation assay. Data represent mean of 3 to 5 mice. Thrombin generation in the absence of emicizumab or FVIII (black dotted line) is the same for both panels. Data on lag time, ETP, and thrombin peak are presented in Table 1.
Thrombin-generation parameters
| Dose, mg/kg . | n . | Lag time, min . | ETP, nM/min . | Thrombin peak, nM . |
|---|---|---|---|---|
| None | 8 | 2.3 ± 0.3 | 73 ± 19 | 7.6 ± 1.2 |
| FVIII | ||||
| 2.5 | 3 | 2.7 ± 0.2 | 371 ± 106 | 32.7 ± 5.5 |
| 5.0 | 3 | 2.0 ± 0.3 | 622 ± 107 | 58.7 ± 9.1 |
| 7.5 | 3 | 2.0 ± 0.4 | 692 ± 41 | 68.3 ± 2.9 |
| 10 | 3 | 2.2 ± 0.3 | 827 ± 113 | 85.7 ± 11.0 |
| Emicizumab | ||||
| 1.5 | 3 | 3.6 ± 1.2 | 146 ± 78 | 10.8 ± 2.8 |
| 3.0 | 4 | 2.5 ± 0.4 | 374 ± 107 | 27.5 ± 9.3 |
| 5.0 | 3 | 2.5 ± 0.4 | 349 ± 111 | 33.0 ± 9.2 |
| 10 | 5 | 1.9 ± 0.2 | 511 ± 82 | 42.2 ± 5.1 |
| Dose, mg/kg . | n . | Lag time, min . | ETP, nM/min . | Thrombin peak, nM . |
|---|---|---|---|---|
| None | 8 | 2.3 ± 0.3 | 73 ± 19 | 7.6 ± 1.2 |
| FVIII | ||||
| 2.5 | 3 | 2.7 ± 0.2 | 371 ± 106 | 32.7 ± 5.5 |
| 5.0 | 3 | 2.0 ± 0.3 | 622 ± 107 | 58.7 ± 9.1 |
| 7.5 | 3 | 2.0 ± 0.4 | 692 ± 41 | 68.3 ± 2.9 |
| 10 | 3 | 2.2 ± 0.3 | 827 ± 113 | 85.7 ± 11.0 |
| Emicizumab | ||||
| 1.5 | 3 | 3.6 ± 1.2 | 146 ± 78 | 10.8 ± 2.8 |
| 3.0 | 4 | 2.5 ± 0.4 | 374 ± 107 | 27.5 ± 9.3 |
| 5.0 | 3 | 2.5 ± 0.4 | 349 ± 111 | 33.0 ± 9.2 |
| 10 | 5 | 1.9 ± 0.2 | 511 ± 82 | 42.2 ± 5.1 |
Thrombograms are depicted in Figure 2B-C.
Dose-response FVIII in a tail-clip–bleeding model
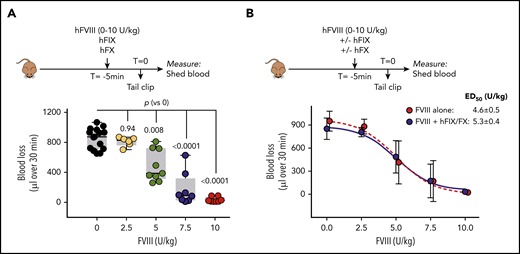
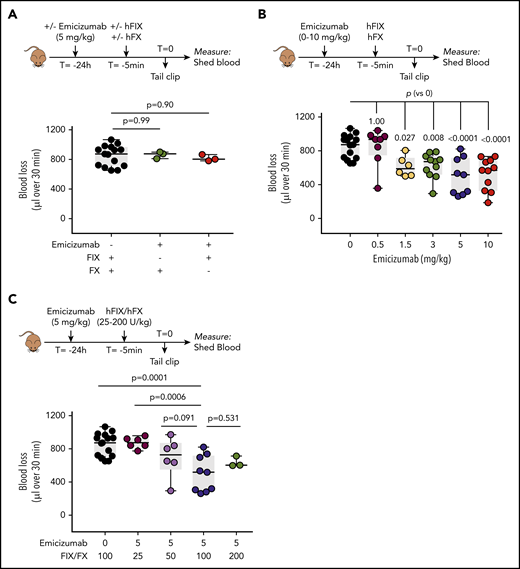
To compare emicizumab with FVIII in the tail-clip model, we first performed a dose-response study using FVIII. Mice received 2.5 to 10 U of FVIII per kilogram, resulting in plasma levels of 5 to 20 U of FVIII per deciliter (not shown). To remain completely in line with mice receiving the FIX/FX/emicizumab cocktail, mice were given FVIII in combination with human FIX/FX. To evaluate whether the presence of additional human FIX and FX would affect the activity of FVIII, a similar dose response was also performed in the absence of human FIX and FX. Five minutes after infusion of FVIII (with or without human FIX/FX), the tail tip was amputated. Mice receiving only human FIX/FX displayed a strong bleeding tendency (blood loss, 852 ± 139 μL; mean plus or minus SD; n = 15; Figure 3A). This amount was unaffected upon infusion of the lowest FVIII dose (2.5 U/kg: 809 ± 61 μL; n = 6; P = .979). However, incremental doses of FVIII (5-7.5-10 U/kg) induced a dose-dependent decrease in blood loss (Figure 3A). No statistical difference in blood loss was observed for the 7.5 and 10 U/kg dose (174 ± 219 μL and 34 ± 34 μL; n = 8; P = .389), suggesting that blood loss is maximally corrected. The half-maximal effective dose was calculated to be 5.3 U/kg (Figure 3B). As depicted in Figure 3B, a similar dose response was observed in the absence of human FIX and FX, with the half-maximal effective dose being 4.6 ± 0.5 U/kg (P = .279 compared with the presence of human FIX/FX).
In vivo dose response of FVIII in FVIII-deficient mice. (A) FVIII-deficient mice received a mixture of FVIII (0-10 U/kg), human FIX (100 U/kg), and human FX (100 U/kg) via retro-orbital infusion. Five minutes after infusion, the terminal 3 mm of the tail was amputated, and shed blood was collected for 30 minutes. Presented is blood loss over 30 minutes vs FVIII dose. Each data point represents an individual mouse. Statistical analysis was performed via 1-way ANOVA with Dunnett multiple comparison. (B) Data presented in panel A but depicted as mean plus or minus SD (n = 6-9; blue symbols). A similar dose-response curve was generated in the absence of human FIX and FX (n = 6 per dose; red symbols). ED50, half-maximal effective dose.
In vivo dose response of FVIII in FVIII-deficient mice. (A) FVIII-deficient mice received a mixture of FVIII (0-10 U/kg), human FIX (100 U/kg), and human FX (100 U/kg) via retro-orbital infusion. Five minutes after infusion, the terminal 3 mm of the tail was amputated, and shed blood was collected for 30 minutes. Presented is blood loss over 30 minutes vs FVIII dose. Each data point represents an individual mouse. Statistical analysis was performed via 1-way ANOVA with Dunnett multiple comparison. (B) Data presented in panel A but depicted as mean plus or minus SD (n = 6-9; blue symbols). A similar dose-response curve was generated in the absence of human FIX and FX (n = 6 per dose; red symbols). ED50, half-maximal effective dose.
Dose-response emicizumab in a tail-clip–bleeding model
We next analyzed mice infused with emicizumab. Mice receiving emicizumab with human FIX or FX alone, but not both, displayed similar blood loss as mice receiving FIX/FX without emicizumab (862 ± 47 μL [n = 3; P = 1.0] and 817 ± 44 μL [n = 3; P = .99] vs 852 ± 139 μL [n = 15]; Figure 4A). Similar blood loss was also observed for the lowest dose of emicizumab (0.5 mg/kg) tested in the presence of human FIX and FX (849 ± 221 μL; n = 8; P = 1.0; Figure 4B). In contrast, a significant reduction in blood loss was observed in mice receiving any emicizumab dose between 1.5 mg/kg and 10 mg/kg (Figure 4B). Interestingly, blood loss was not significantly different between these emicizumab doses tested, that is, 1.5, 3, 5, and 10 mg/kg (P = .34). Furthermore, the bleeding profiles were also similar between these doses of emicizumab (supplemental Figure 1, available on the Blood Web site).
Emicizumab activity in FVIII-deficient mice. (A) FVIII-deficient mice were given incomplete combinations of emicizumab (3 mg/kg given 24 hours before tail amputation), FIX (100 U/kg given 5 minutes before tail amputation), and FX (100 U/kg given 5 minutes before tail amputation), each time with 1 of the 3 proteins missing. The tail bleeding was executed as described in the Figure 3 legend. Data represent individual mice. (B) FVIII-deficient mice were given emicizumab (0-10 mg/kg given 24 hours before tail amputation) and FIX/FX (both 100 U/kg given 5 minutes before tail amputation). Presented is blood loss over 30 minutes vs emicizumab dose. Data represent individual mice. Data for 0 mg/kg emicizumab are similar as those presented in Figure 3A. (C) Emicizumab (5 mg/kg) was given to FVIII-deficient mice in combination with different doses of FIX and FX (25-200 U/kg) prior to tail bleeding as described for panel B. Data for 0 mg/kg emicizumab are similar to those presented in Figure 3A and data for 100 U FIX-FX/kg are similar to those presented in panel B. Statistical analysis was performed via 1-way ANOVA with Dunnett multiple comparison.
Emicizumab activity in FVIII-deficient mice. (A) FVIII-deficient mice were given incomplete combinations of emicizumab (3 mg/kg given 24 hours before tail amputation), FIX (100 U/kg given 5 minutes before tail amputation), and FX (100 U/kg given 5 minutes before tail amputation), each time with 1 of the 3 proteins missing. The tail bleeding was executed as described in the Figure 3 legend. Data represent individual mice. (B) FVIII-deficient mice were given emicizumab (0-10 mg/kg given 24 hours before tail amputation) and FIX/FX (both 100 U/kg given 5 minutes before tail amputation). Presented is blood loss over 30 minutes vs emicizumab dose. Data represent individual mice. Data for 0 mg/kg emicizumab are similar as those presented in Figure 3A. (C) Emicizumab (5 mg/kg) was given to FVIII-deficient mice in combination with different doses of FIX and FX (25-200 U/kg) prior to tail bleeding as described for panel B. Data for 0 mg/kg emicizumab are similar to those presented in Figure 3A and data for 100 U FIX-FX/kg are similar to those presented in panel B. Statistical analysis was performed via 1-way ANOVA with Dunnett multiple comparison.
Because emicizumab activity is restricted by the concentrations of FIXa and FX available,5 we tested the emicizumab-mediated response at different doses of FIX and FX. Compared with the dose of 100 U/kg used throughout the study, no differences were observed with regard to doses of 50 and 200 U/kg: 700 ± 235 μL (P = .091) for 50 U/kg and 639 ± 65 μL (P = .531) for 200 U/kg, compared with 498 ± 217 μL for 100 U/kg (Figure 4C). In contrast, significantly more blood loss was observed at a dose of 25 U/kg (874 ± 67 μL; P = .0006 compared with 100 U/kg), an amount similar to that of vehicle-treated mice (852 ± 139 μL).
Together, these data show that this model may be used to analyze emicizumab-mediated hemostasis in an unbiased manner, and emicizumab shows relevant cofactor-like activity under these in vivo conditions. By using the dose-response curve for FVIII as a reference, we used the average amount of blood loss from emicizumab-treated mice (1.5-10 mg/kg) to calculate FVIII equivalence in this model. The combined average blood loss was 566 ± 177 μL (n = 37), and interpolating this amount into the FVIII-reference curve revealed a FVIII equivalence of 4.5 ± 1.3 U/kg (which relates to an FVIII plasma concentration of 9.0 ± 0.3 U/dL). The bleeding profiles of the mice receiving these emicizumab doses were similar to those of the 5 U/kg FVIII dose (supplemental Figure 1).
Combining emicizumab with FVIII
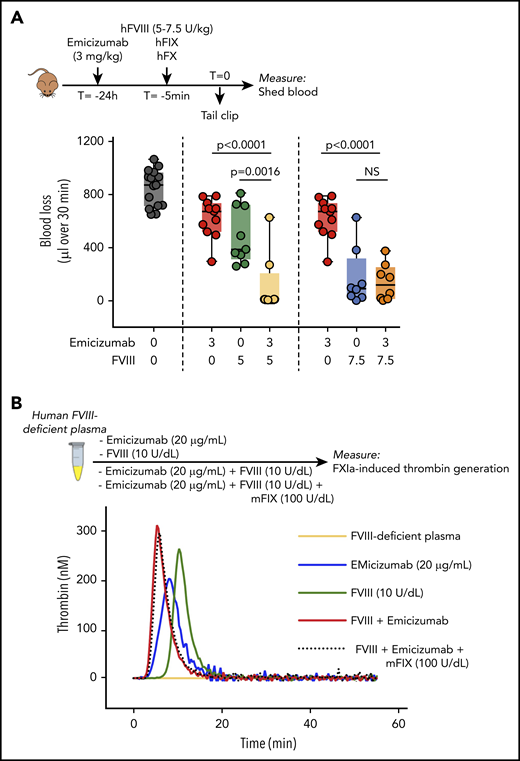
Compared with emicizumab, FVIII has a superior affinity for FIXa,5 and it is often assumed that FVIII will outcompete emicizumab during coagulation when both FVIII and emicizumab are present. We investigated, therefore, how hemostasis in FVIII-deficient mice is affected when FVIII and emicizumab are applied together. As shown in Figures 3 and 4, a single dose of 5 U of FVIII per kilogram or 3 mg of emicizumab per kilogram is insufficient to fully correct bleeding. We therefore tested what would happen if both were given simultaneously (Figure 5). Interestingly, when a FVIII dose of 5 U/kg was combined with an emicizumab dose of 3 mg/kg, blood loss was corrected to the same extent as observed for an FVIII dose of 7.5 U/kg or higher (Figure 5A). No further reduction in blood loss was observed when emicizumab was combined with a dose of 7.5 U of FVIII per kilogram (Figure 5A). A potential pitfall of this experiment is that FVIII would be able to associate with both human and murine FIXa, whereas emicizumab is restricted to its interaction with human FIXa. To test whether this would affect the outcome, we designed an in vitro FIXa-induced thrombin-generation experiment using human FVIII-deficient plasma, to which we added purified recombinant murine FIX (Figure 5B). Compared with emicizumab (20 μg/mL) or FVIII (0.1 U/mL) alone, thrombin generation was increased when combining FVIII and emicizumab (Figure 5B). The addition of murine FIX (1 U/mL) to the mixture containing FVIII and emicizumab did not further modify thrombin generation. This supports the view that the presence of both human and murine FIXa does not affect the additive activity of FVIIIa and emicizumab. Together, these results show that emicizumab may provide additional hemostatic support to the tenase complex at low FVIII concentrations.
Combined activity of FVIII and emicizumab. (A) Emicizumab (3 mg/kg given 24 hours before tail bleeding) and FVIII (5 or 7.5 U/kg given 5 minutes before tail bleeding) were given separately or in combination together with human FIX/FX (100 U/kg) to FVIII-deficient mice as described for Figures 3 and 4. Each data point represents an individual mouse. Data for FVIII without emicizumab are similar to those presented in Figure 3A, and data for emicizumab without FVIII are similar to those presented in Figure 4B. Statistical analysis was performed via 1-way ANOVA with Tukey multiple comparison. Data of mice receiving only FIX/FX (but no emicizumab or FVIII) are similar as those presented in Figure 3A. (B) FXIa-induced thrombin generation of human FVIII-deficient plasma (orange), which was spiked with emicizumab (20 μg/mL; blue), FVIII (10 U/dL; green), or both in the absence (red) or presence (black dotted line) of recombinant murine FIX (mFIX; 100 U/dL). Thrombogram is representative for 3 independent experiments. NS, not significant.
Combined activity of FVIII and emicizumab. (A) Emicizumab (3 mg/kg given 24 hours before tail bleeding) and FVIII (5 or 7.5 U/kg given 5 minutes before tail bleeding) were given separately or in combination together with human FIX/FX (100 U/kg) to FVIII-deficient mice as described for Figures 3 and 4. Each data point represents an individual mouse. Data for FVIII without emicizumab are similar to those presented in Figure 3A, and data for emicizumab without FVIII are similar to those presented in Figure 4B. Statistical analysis was performed via 1-way ANOVA with Tukey multiple comparison. Data of mice receiving only FIX/FX (but no emicizumab or FVIII) are similar as those presented in Figure 3A. (B) FXIa-induced thrombin generation of human FVIII-deficient plasma (orange), which was spiked with emicizumab (20 μg/mL; blue), FVIII (10 U/dL; green), or both in the absence (red) or presence (black dotted line) of recombinant murine FIX (mFIX; 100 U/dL). Thrombogram is representative for 3 independent experiments. NS, not significant.
Discussion
Given the impact that emicizumab has on the management of hemophilia A, it is surprising that accessible animal models are thus far lacking. This limitation is mostly due to the species-restricted interaction between emicizumab and its ligands, FIX and FX. To fill this gap, we here present a simple and straightforward mouse model, which allows for testing of emicizumab in vivo. The model is based on the IV infusion of emicizumab in combination with human FIX and FX. This combination indeed results in a significant reduction in blood loss compared with vehicle-treated FVIII-deficient mice, at least when doses between 1.5 and 10 mg/kg emicizumab are being used.
A single IV infusion of emicizumab 24 hours before the tail-clip intervention results in reproducible and dose-dependent levels of the antibody (Figure 1). FIX and FX were dosed such that their plasma levels were within the normal range (0.85 U/dL and 1.0 U/dL, respectively). Based on the respective emicizumab, FIX, and FX concentrations used in this study, the amount of circulating ternary complex varies between 128 pM for the lowest emicizumab concentration and 1270 pM for the highest emicizumab concentration (Figure 1C). Higher emicizumab concentrations would not lead to increased levels of ternary complex (red dots in Figure 1C), and could even lead to reduced complex formation. This bell-shaped curve is due to the fact that if the molar excess of emicizumab increases, there will be fewer emicizumab molecules that will bind both FIX and FX simultaneously.
Although emicizumab levels are expected to remain relatively stable during the 30-minute tail-clip intervention because of its long half-life, this is less so for concentrations of human FIX and FX. FIX and FX have half-lives that exceed 3 hours in mice,17,18 and it is thus anticipated that <30% of FIX and FX will be removed during the 30-minute experiment. To test whether the amounts of FIX and FX that remain would be sufficient, we performed experiments in which mice were given one-half the amount of FIX and FX, that is, 50 U/kg instead of 100 U/kg. Blood loss was similar between 50 and 100 U/kg doses (Figure 4). This suggests that when giving a 100 U/kg dose, and even if 50% of FIX and FX would be eliminated during the 30-minute observation period, there would still be sufficient amounts of FIX and FX remaining to mediate emicizumab-dependent hemostasis.
The opposite was true when FIX and FX were given a 25 U/kg dose, with blood loss being similar to that of vehicle-treated mice (Figure 4). Apparently, a minimal amount of FIX and FX is needed to support emicizumab activity. By using the calculation for ternary complexes, it appears that a minimum of 260 pM ternary complex is needed to achieve a reduction in blood loss, at least in this model (Table 2). It is interesting to speculate on the consequences of these data in view of individuals with reduced FIX and FX levels, in particular newborns. Newborns have FIX and FX levels that are reduced 40% to 60% during the first month of their life.19,20 These levels restrict ternary complex formation, and will remain below 250 pM if the clinical therapeutic dose of 5.5 mg/dL is being used. It is possible, therefore, that emicizumab will be less efficient in these patients.
Relation ternary complex formation and hemostatic potential
| Emicizumab, mg/kg . | FIX, U/kg . | FX, U/kg . | Estimated ternary complex, pM . | Reduced blood loss? . |
|---|---|---|---|---|
| 0 | 100 | 100 | 0 | No |
| 5 | 0 | 100 | 0 | No |
| 5 | 100 | 0 | 0 | No |
| 0.5 | 100 | 100 | 129 | No |
| 1 | 100 | 100 | 333 | Yes |
| 3 | 100 | 100 | 748 | Yes |
| 5 | 100 | 100 | 1001 | Yes |
| 10 | 100 | 100 | 1270 | Yes |
| 5 | 25 | 25 | 66 | No |
| 5 | 50 | 50 | 258 | Yes |
| 5 | 200 | 100 | 1961 | Yes |
| Emicizumab, mg/kg . | FIX, U/kg . | FX, U/kg . | Estimated ternary complex, pM . | Reduced blood loss? . |
|---|---|---|---|---|
| 0 | 100 | 100 | 0 | No |
| 5 | 0 | 100 | 0 | No |
| 5 | 100 | 0 | 0 | No |
| 0.5 | 100 | 100 | 129 | No |
| 1 | 100 | 100 | 333 | Yes |
| 3 | 100 | 100 | 748 | Yes |
| 5 | 100 | 100 | 1001 | Yes |
| 10 | 100 | 100 | 1270 | Yes |
| 5 | 25 | 25 | 66 | No |
| 5 | 50 | 50 | 258 | Yes |
| 5 | 200 | 100 | 1961 | Yes |
Observing that a low dose of FVIII, in itself too low to fully correct the bleeding, combined with emicizumab resulted in a complete correction of blood loss was of interest (Figure 5). Apparently, there is an additive effect of both components under these conditions. One could argue that FVIIIa has a much higher affinity than emicizumab for FIXa, and will thus outcompete emicizumab. Nevertheless, it is reasonable to assume that the amount of FVIIIa available (in our case 0.03 nM, if all 10 U of FVIII per deciliter are being activated) is much less than the amount of FIXa. Assuming that 5% of FIXa would be activated, then there is 3.8 nM human FIXa present in our experiments; if 0.03 nM of these interactswith FVIIIa, there would be 3.77 nM left for the interaction with emicizumab. In other words, complex formation with emicizumab will be virtually unchanged. Indeed, we noticed that doubling the FIX concentration available for FVIII did not affect thrombin generation in the presence of a low dose of FVIII and emicizumab (Figure 5B).
Our proposed model is, like many other animal models, not without limitations. First, in contrast to the human system, only FIX and FX are of human origin in this mouse model. Moreover, murine FIX and FX are still present. The murine and human proteins may behave differently during the hemostatic process. It should be noted that both human FIX and FX are able to correct the deficiencies of their murine orthologs in the respective mouse knockout models,21,22 suggesting that the proteins are fully functional in the murine context. We analyzed this further in a diluted aPTT assay, in which we compared the clotting times of murine plasma containing only murine FIX with murine plasma containing both human and murine FIX (supplemental Figure 2). A similar dose response was observed, suggesting that both human and murine FIX are reacting similarly in this assay.
A second limitation is that the functionality of emicizumab has been tested only in the tail-clip-model. Emicizumab activity is driven by the amount of FIXa generated, and this amount may be different dependent on the severity of the injury and the location of the injury. We are currently exploring the hemostatic potential of emicizumab in other models with injuries at different locations and with different severities.
Finally, as mentioned earlier in “Discussion,” levels of human FIX and FX are transient, and do not allow for long-term follow-up. However, there are strategies for obtaining stable levels over a longer period of time. Attempts to generate knock-in mice that express human FIX and FX have only been partially successful, as these mice express strongly reduced levels of both proteins (10% to 20%).23 These levels are too low to form sufficient amounts of ternary emicizumab complexes. Alternatively, FIX and FX can be produced over a longer period of time following hydrodynamic injection (∼2 weeks stable production) or by using viral vectors (eg, adeno-associated virus or lentivirus). The issue here is that the protein expression levels are often highly variable and difficult to predict. However, our data using different doses of FIX and FX indicate that levels of FIX (85 U/dL) and FX (100 U/dL) can be halved or doubled without any major consequences, providing a relatively extended window of expression for which to aim.
In conclusion, we provide a relatively straightforward and robust mouse model that can be used to assess the in vivo action of emicizumab (or variants thereof) alone or in combination with other procoagulant agents.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The work was supported by the Societé Française d’Hématologie (SFH) and the Agence Nationale de la Recherche (ANR-14-OHRI-0013 and ANR-18-CE17-0010-01).
Authorship
Contribution: S.F., V.M., and C.K. performed experiments; S.F., I.P., C.C., O.D.C., P.J.L., and C.V.D. analyzed data; O.D.C., A.N., P.J.L., and C.V.D. conceived the study; A.K., O.D.C., P.J.L., and C.V.D. supervised the study; P.J.L. and C.V.D. wrote the manuscript; and all authors contributed to the editing of the final manuscript.
Conflict-of-interest disclosure: P.J.L. received honoraria/speaker’s fees from Biotest, Chugai, Roche, Sobi, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Peter J. Lenting, U1176, INSERM, 80 rue du General Leclerc, 94276 Le Kremlin-Bicêtre cedex, France; e-mail: peter.lenting@inserm.fr.
REFERENCES
Author notes
S.F., I.P., and O.D.C. contributed equally to the study.
P.J.L. and C.V.D. contributed equally to the study.
Requests for data sharing should be made to the corresponding author (peter.lenting@inserm.fr).