TO THE EDITOR:
Hepatitis B virus (HBV) reactivation typically occurs in patients with B-cell malignancies and concomitant HBV infection after exposure to immunosuppressive or cytotoxic agents.1-4 These agents, commonly used in the treatment of B-cell malignancies, such as B-cell–depleting antibodies, anthracycline derivatives, high-dose corticosteroids, immune checkpoint inhibitors, tyrosine-kinase inhibitors, and antimetabolites, are associated with varied risks of HBV reactivation.2,4-10 When administering these agents, routine HBV screening, HBV-DNA monitoring, and prophylactic or preemptive nucleotide analogue treatment are recommended to prevent this potentially fatal complication.4,11-13
Chimeric antigen receptor–engineered (CAR) T-cell therapy represents one of the most promising fields in cancer research and has yielded unprecedented responses in B-cell malignancies.14-19 However, when the durable B-cell depletion induced by functional CAR T cells is considered, patients with HBV infection are at high or even very high risk for HBV reactivation after CAR T-cell infusion, particularly functional CAR T cells, which cannot be withdrawn in case of HBV reactivation. To circumvent HBV reactivation–related hepatitis and mortality, almost all the clinical trials of CAR T-cell therapy exclude patients with HBV infection. Thus, the safety of CAR T-cell therapy and the prevention of HBV reactivation should be evaluated in this specific patient population. Moreover, HBV infection may negatively affect the clinical outcome and therapeutic response to standard treatment in B-cell malignancies.20,21 The impact of HBV infection on the efficacy of CAR T-cell therapy should also be elucidated, especially in hyperendemic Asia-Pacific regions.22,23
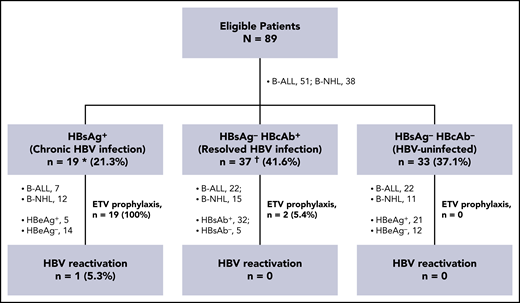
In the first issue of Blood in 2020, we reported the safety and efficacy of anti-CD19 and anti-CD22 CAR (CAR19/22) in a T-cell combination-treatment trial (Chinese Clinical Trial Registry, #ChiCTR-OPN-16008526) in 89 eligible patients who had refractory/relapsed B-cell acute lymphoblastic leukemia (B-ALL) or B-cell non-Hodgkin lymphoma (B-NHL).24 Among the 89 patients enrolled from March 2016 through January 2018, 19 (21.3%) had chronic HBV infection (defined as serologically positive for hepatitis B surface antigen [HBsAg]) and 37 patients (41.6%) had resolved HBV infection (defined as HBsAg−, but positive for antibodies against hepatitis B core antigen [HBcAb]) (Figure 1; supplemental Table 1, available on the Blood Web site). Baseline features of these patients were balanced across different HBV serological subgroups (supplemental Table 2). Among patients with resolved HBV infection, 32 (86.5%) were seropositive for antibodies against hepatitis B surface antigen (HBsAb). We retrospectively reviewed HBV reactivation in these patients and preliminarily explored the impact of HBV infection on CAR T-cell therapy. HBV reactivation was defined as a ≥100-fold increase in HBV DNA when compared with baseline level or HBV DNA ≥103 IU/mL in a patient with a previously undetectable level or reverse seroconversion from HBsAg− to HBsAg+.13 The cutoff date for data collection was 31 March 2019.24
Retrospective analysis of HBV reactivation in patients with different HBV serologic statuses. Among the 89 patients who had received a diagnosis of B-ALL or B-NHL, 21 received ETV prophylaxis, including all of the HBsAg+ patients and 2 of the HBsAg−/HBcAb+ patients. These 2 patients were also seropositive for HBsAb. HBV reactivation was detected in 1 patient with chronic HBV infection. Except for this patient, no patient in each subgroup had HBV reactivation, severe hepatitis, or HBV-related mortality. *Includes 1 patient seropositive for anti-HCV IgG, in whom HCV-RNA was undetectable. †Includes 1 patient seropositive for anti-HEV IgG, but negative for anti-HEV IgM. HCV, hepatitis C virus; HEV, hepatitis E virus.
Retrospective analysis of HBV reactivation in patients with different HBV serologic statuses. Among the 89 patients who had received a diagnosis of B-ALL or B-NHL, 21 received ETV prophylaxis, including all of the HBsAg+ patients and 2 of the HBsAg−/HBcAb+ patients. These 2 patients were also seropositive for HBsAb. HBV reactivation was detected in 1 patient with chronic HBV infection. Except for this patient, no patient in each subgroup had HBV reactivation, severe hepatitis, or HBV-related mortality. *Includes 1 patient seropositive for anti-HCV IgG, in whom HCV-RNA was undetectable. †Includes 1 patient seropositive for anti-HEV IgG, but negative for anti-HEV IgM. HCV, hepatitis C virus; HEV, hepatitis E virus.
A total of 21 patients received entecavir (ETV) prophylaxis, including all 19 HBsAg+ patients and 2 (5.4%) who were HBsAg−/HBcAb+ (Figure 1; supplemental Table 1). Those 2 patients were also seropositive for HBsAb, which is associated with a reduced, but not eliminated, risk of reactivation.25 Prophylactic ETV was initiated before CAR T-cell infusion with a median interval of 14 (interquartile range [IQR], 8-23) days and was maintained for a median duration of 15.6 (IQR, 6.3-22.2) months until the last follow-up. B-cell recovery was detected in 17 patients. The median time of ETV prophylaxis was sustained for 7.9 (IQR, 2.8-13.3) months after B-cell recovery.
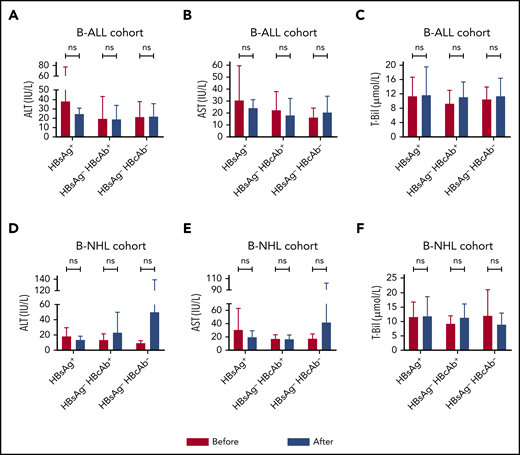
Within the first month after CAR T-cell infusion, no statistically significant increases in serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (T-Bil) were found in both disease cohorts, regardless of HBV infection status (Figure 2). The reported incidence of HBV reactivation in patients undergoing immunochemotherapy ranged from 26% to 53% in patients with chronic HBV infection and varied from 3% to 41.5% in patients with resolved HBV infection.1-4,7,13 In the present study, HBV reactivation was detected in 1 (5.3%) patient with chronic HBV infection, 4 months after CAR T-cell infusion (Figure 1; supplemental Table 1). Except for this patient, no patient in either subgroup experienced HBV reactivation or severe hepatitis (more than five times the upper limit of normal of aminotransferase or more than three times the upper limit of normal of bilirubin), or died of HBV.
Comparable concentration changes in serum ALT, AST, and T-Bil after CAR T-cell infusion in patients with various HBV serologic statuses. The variations from the baseline levels to the peak concentrations of ALT (A,D), AST (B,E), and T-Bil (C,F) within the first month were similar in the disease cohorts, regardless of HBV infection status. ns, not significant; Before, before CAR T-cell infusion; After, after CAR T-cell infusion.
Comparable concentration changes in serum ALT, AST, and T-Bil after CAR T-cell infusion in patients with various HBV serologic statuses. The variations from the baseline levels to the peak concentrations of ALT (A,D), AST (B,E), and T-Bil (C,F) within the first month were similar in the disease cohorts, regardless of HBV infection status. ns, not significant; Before, before CAR T-cell infusion; After, after CAR T-cell infusion.
The patient who experienced HBV reactivation was a 70-year-old woman who had received a diagnosis of diffuse large B-cell lymphoma 22 months before enrollment. She had a known history of chronic hepatitis B and was seropositive for HBsAg, HBcAb, and antibody against the hepatitis B e-antigen (HBeAb), but with unquantifiable HBV-DNA copy number (<100 IU/mL) and normal ALT and AST levels at enrollment. ETV prophylaxis started (0.5 mg/d orally) 28 days before CAR T-cell infusion and was maintained thereafter. The patient had sustained B-cell aplasia after CAR T-cell infusion and achieved durable complete remission at month 3. Unfortunately, she died of septic shock 4 months after CAR T-cell infusion. At that time, HBV DNA copies had increased to 3.62 × 104 IU/mL, and severe hepatitis had manifested (ALT, 161 U/L; AST, 749 U/L; and T-Bil, 74.6 μmol/L). HBV reactivation was reported in 2 patients at 1.5 and 3 months, after self-discontinuation of ETV.6,8 For the first time, we describe a patient with chronic HBV infection in whom HBV reactivation occurred 4 months after CAR T-cell infusion, even with continuous ETV prophylaxis. Therefore, HBV DNA should still be closely monitored in patients who are receiving antiviral prophylaxis after CAR T-cell infusion, especially in those who have a history of lamivudine resistance. When virologic breakthrough (10-fold increase in serum HBV DNA from nadir) during ETV or adefovir treatment is suspected or confirmed, a test for antiviral-resistant mutants and a switch to tenofovir is recommended.4,13
Furthermore, when compared with HBV-uninfected patients (defined as seronegative for both HBsAg and HBcAb), patients with chronic or resolved HBV infection had similar peak concentrations of serum interleukin-6 (sIL-6) and C-reactive protein (supplemental Figure 1A-D) and comparable severities of cytokine release syndrome and CAR T-cell–related encephalopathy syndrome (supplemental Figure 1E-H) in both disease cohorts. Although higher sIL-6 was observed in 3 patients with chronic HBV or hepatitis C infection,8 our data suggest that chronic or resolved HBV infection did not affect the production of sIL-6 or serum C-reactive protein and showed no impact on the risk of cytokine release syndrome or CAR T-cell–related encephalopathy syndrome during CAR T-cell therapy. Meanwhile, no statistically significant difference was demonstrated in either progression-free or overall survival in patients with B-ALL or B-NHL, regardless of HBV serologic status (supplemental Figure 2A-D).
Taken together, these results indicate that ETV prophylaxis is essential and effective for the prevention of HBV reactivation in patients with chronic HBV infection who are undergoing CAR T-cell therapy, and chronic or resolved HBV infection may not affect the safety and efficacy of CAR T-cell therapy. Given the retrospective nature of this study, validation in multicenter prospective trials is warranted. The proper duration of antiviral prophylaxis and the prevention strategy in patients with resolved HBV infection in CAR T-cell therapy are still unclear and should be further investigated. With reference to the reported data and the recommendations for managing HBV reactivation,4,6-8,13 we suggest that antiviral prophylaxis be administered in patients with chronic HBV infection before lymphodepletion chemotherapy and then be maintained for at least 12 months after B-cell recovery. Levels of HBV DNA should be further monitored by real-time polymerase chain reaction assay for at least 6 months, even after the withdrawal of antiviral prophylaxis. HBV DNA-monitoring–guided preemptive antiviral therapy could be conducted in patients with resolved HBV infection.4,7
The online version of this article contains a data supplement.
All published data and material are available upon request from the corresponding author.
Acknowledgments
The authors thank all the faculty and staff of the Clinical and Laboratory Unit of the Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, for clinical and technical support; and Wuhan Bio-Raid Biotechnology Co., Ltd., for service in cell manufacturing and quality control.
This work is supported by Key Program of the National Natural Science Foundation of China grant 81830008 (J.Z.); the National Natural Science Foundation of China grants 81670152 (Liang Huang), 81600120, (N.W.), and 81873427 (J.W.); Natural Science Foundation of Hubei Province grants 2018ACA140 and 2016CFA011 (J.Z.), Huanghe Talents Plan of Wuhan City grant HHYC-2015002 (J.Z.), National High Technology Research and Development Program of China 863, grant 2014AA020532 (Liang Huang), the Milstein Medical Asian American Partnership Foundation (2018 MMAAP Foundation Hematology Fellowship Award) (Liang Huang) and Applied Basic Research Project of Wuhan City grant 2017060201010156 (Y.X.).
Authorship
Contribution: J.Z. and Liang Huang designed and supervised the clinical study; T.Z., C.G., and S.Z. supervised CAR T-cell production; N.W., W.C., H.X., and M.X. conducted preclinical validation and quality control; N.W., W.C., J.W., Lifang Huang, and H.X. collected clinical data; Liang Huang, N.W., and J.W. analyzed the data and wrote and revised the manuscript; N.W. and Liang Huang performed statistical analyses; and J.Z., Liang Huang, C.L., Y.X., Y.C., Y.Z., J.W., D.L., and N.W. enrolled the patients and took care of them.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jianfeng Zhou, Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095 Jiefang Ave, Wuhan, Hubei, China; e-mail: jfzhou@tjh.tjmu.edu.cn; and Liang Huang, Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095 Jiefang Ave, Wuhan, Hubei, China; e-mail: lhuang@tjh.tjmu.edu.cn.
REFERENCES
Author notes
W.C. and J.W. contributed equally to this study.