Abstract
The success of allogeneic hematopoietic cell transplantation depends heavily on the delicate balance between the activity of the donor immune system against malignant and nonmalignant cells of the recipient. Abrogation of alloreactivity will lead to disease relapse, whereas untamed allo-immune responses will lead to lethal graft-versus-host disease (GVHD). A number of cell types have been identified that can be used to suppress alloreactive immune cells and prevent lethal GVHD in mice. Of those, mesenchymal stromal cells and, to a lesser extent, regulatory T cells have demonstrated efficacy in humans. Ideally, cellular therapy for GVHD will not affect alloreactive immune responses against tumor cells. The importance of tissue damage in the pathophysiology of GVHD rationalizes the development of cells that support tissue homeostasis and repair, such as innate lymphoid cells. We discuss recent developments in the field of cellular therapy to prevent and treat acute and chronic GVHD, in the context of GVHD pathophysiology.
Introduction
Although allogeneic hematopoietic cell transplantation (HCT) is a highly effective form of cancer immunotherapy, its effectiveness is often dwarfed by its side effects, most importantly acute and chronic graft-versus-host disease (GVHD) and opportunistic infections. The majority of allogeneic HCT recipients will at 1 point experience GVHD despite preventive measures, and 10% to 30% of allogeneic HCT recipients succumb to transplantation-related complications, most of which are directly or indirectly related to GVHD.1
First-line therapy for acute and chronic GVHD consists of topical or systemic corticosteroids. When steroids fail, there are only a few alternatives available. This is in part inherent to the main purpose of allogeneic HCT as cancer immunotherapy, which precludes complete abrogation of donor immune responses as a therapy for GVHD because it will eliminate the graft-versus-tumor effect and lead to disease relapse. In addition, intensifying immune suppression will increase the risk for opportunistic infections, as exemplified by the high infection-related mortality reported for steroid-refractory acute GVHD treated with the pan-lymphocyte depleting antithymocyte globulin.2 Less aggressive alternatives such as the janus kinase 2 inhibitor ruxolitinib, the tyrosine kinase inhibitors imatinib and ibrutinib, and many others are under investigation.3,4 Cellular therapy, in most cases as advanced therapy medicinal product, provides an interesting alternative.
Pathophysiology of GVHD
GVHD can be defined as an inflammatory disease caused by unrestrained activity of the donor immune system against nonmalignant cells and tissues of the allogeneic HCT recipient. GVHD is categorized as acute or chronic, depending on the time of onset relative to the transplantation and on clinical characteristics. Acute GVHD typically presents as an acute inflammatory syndrome involving the skin, liver and/or gastrointestinal tract, whereas chronic GVHD can affect any organ and is characterized by a gradual onset and fibrotic inflammation. Alloreactivity of the donor immune system toward the recipients’ malignant and nonmalignant cells represents a continuum, with acute GVHD often blending into chronic GVHD, and graft-versus-leukemia/lymphoma (GVL) and GVHD responses intimately linked.
Acute and chronic GVHD pathophysiology rests on 3 pillars: tissue damage, impaired tissue homeostasis and repair, and alloreactivity (Figure 1).5 In murine acute GVHD models, damage to host tissues is inflicted typically by conditioning radiotherapy, after which a complete or partially mismatched donor immune system is introduced that leads to allo-immune inflammation and more damage. In human allogeneic HCT recipients, damage can be caused by many different ways: conditioning chemotherapy and radiotherapy, viruses, bacteria, sunburn, wounds, and others. Regardless of the cause, tissue damage leads to the release of damage-associated molecular patterns (DAMPs), and, via damaged barrier tissues, to the entrance of pathogens and pathogen-associated molecular patterns into the system. DAMPs and pathogen-associated molecular patterns prime and activate innate immune cells such as neutrophils and macrophages, which subsequently activate the adaptive immune system. In this pro-inflammatory milieu, alloreactive adaptive immune cells are easily activated to become reactive against non-self-expressing cells, leading to GVHD and GVL immunity.5 In the absence of normal tissue repair mechanisms, a vicious cycle emerges, with damage leading to immune activation, resulting in more damage and inflammation.6
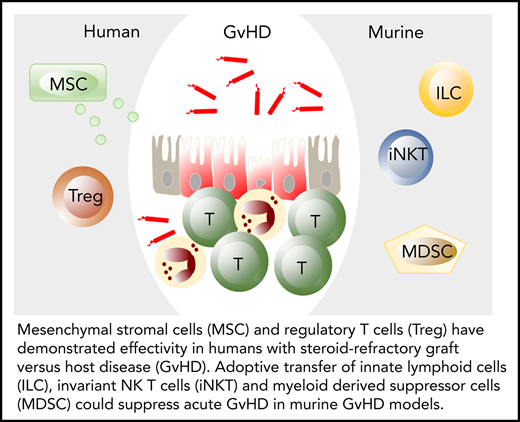
Three key elements of GVHD pathophysiology: tissue damage, impaired repair, and alloreactvity. Typically, tissue damage is inflicted during conditioning chemotherapy and radiotherapy, at a time when tissue repair mechanisms are most impaired. However, tissue damage can occur at any time, for example, as a result of viral infection or viral reactivation. In the presence of alloreactive immune cells and in the absence of tissue repair mechanisms, this can lead to GVHD. B, B cells; T, T cells. Blue symbols: microbiome.
Three key elements of GVHD pathophysiology: tissue damage, impaired repair, and alloreactvity. Typically, tissue damage is inflicted during conditioning chemotherapy and radiotherapy, at a time when tissue repair mechanisms are most impaired. However, tissue damage can occur at any time, for example, as a result of viral infection or viral reactivation. In the presence of alloreactive immune cells and in the absence of tissue repair mechanisms, this can lead to GVHD. B, B cells; T, T cells. Blue symbols: microbiome.
Tissue homeostasis and repair mechanisms are thus important factors in GVHD pathophysiology. Key regulators of tissue homeostasis and repair are the microbiome and the innate immune system.7 Commensal bacteria produce factors such as butyrate and tryptophans, which interact with epithelial cells of the gut. These factors serve as nutrients for epithelial cells and are important in the maintenance of tight cell–cell junctions and thereby epithelial barrier function. Microbiota products also have indirect effects, for example, by serving as activating ligands for innate lymphoid cells (ILC). ILC contribute to tissue homeostasis via their production of interleukin-22 (IL-22), which acts on intestinal epithelial stem cells and Paneth cells.8 Moreover, ILC have immune regulatory capacities and can suppress alloreactive T cells.9,10 Several studies in mice and men have demonstrated that early damage to the microbiome and depletion of the ILC compartment is associated with a predisposition to develop GVHD.11-13
Given the observation that the earliest events in GVHD pathogenesis relate to tissue damage and activation of the donor immune system, the emergence of cellular therapies aimed at suppressing alloreactive immune responses or at preventing tissue damage and harnessing tissue repair harbors great promise. Because of space restrictions, we focus on human data rather than mouse models whenever possible.
Cellular therapy to suppress alloreactive lymphocytes
Mesenchymal stromal cells
The first patient that received cellular therapy for GVHD was a 9-year-old boy with steroid-refractory, hyperacute GVHD.14 He received haploidentical mesenchymal stem cells (MSC) from his mother (not the HCT donor) after which GVHD resolved within a few weeks. MSC are defined by the International Society of Cellular Therapies as cells that: (1) adhere to plastic in standard culture conditions; (2) express CD73, CD90, and CD105 in the absence of CD34, CD45, CD14/CD11b, CD79a/CD19, and HLA-DR; and (3) are able to differentiate in vitro into osteoblasts, adipocytes, and chondroblasts.15 Their powerful regenerative and immunosuppressive capacities and their low immunogenicity makes these cells attractive candidates for cellular therapies for a range of clinical conditions, including GVHD.16,17 A number of retrospective studies on steroid refractory GVHD reported a large variation in the effect of MSC.18,19 In a recent prospective, randomized multicenter study including 260 pediatric and adult patients with steroid-refractory acute GVHD, the addition of ex vivo cultured adult human MSC to standard care showed no difference in durable complete responses compared with placebo, except for pediatric patients and patients with hepatic involvement.20 This study confirmed others showing that patient age and the organ involved significantly associated with responses to MSC.21,22 In another recent study, clinical response assessed as early as 1 week after bone marrow‐derived MSC infusion predicted patients’ overall survival, and MSC nonresponsiveness was associated with a dismal prognosis (2-year survival < 10%).23
The variety in therapeutic efficiency of MSC has been related to a number of factors, including source of MSC, methods of expansion and priming, and intrinsic properties such as the capacity of MSC to migrate to GVHD affected tissues.24-26 Our group demonstrated that the low in vitro migratory behavior of human MSC was due to a laminin-induced increased rigidity of MSC nuclei.27 The route of MSC delivery (eg, intra-arterial, intravenous, locally) has also been considered as a variable that affects MSC therapy outcome, although in a recent analysis this could not be confirmed.28 The observation that MSC from 1 donor can be effective in 1 recipient and ineffective in another suggested that recipient factors play a major role in the mechanism of action of MSC.29 For example, MSC can activate ILC, that have tissue reparative properties, and it can be hypothesized that the presence or absence of ILC in MSC recipients may affect its effect.30 In addition, it was demonstrated that MSC need to undergo apoptosis to be effective in suppressing alloreactivity.31 Apoptotic MSC (dubbed “apoMSC”) were phagocytosed by recipient cells in the lungs of mice with acute GVHD but not in control mice, inducing indoleamine 2,3-dioxygnease (IDO, produced by phagocytes) dependent resolution of GVHD. Importantly, clinical responses to MSC could be predicted by the capacity of peripheral blood derived mononuclear cells of patients with steroid-refractory GVHD to induce apoptosis of MSC ex vivo. Together, these data support the hypothesis that the effectiveness of MSC depends significantly on immunological factors of the MSC recipient.
Despite the large variation in clinical outcomes, MSC therapy is now considered second-line therapy for steroid-refractory GVHD in most centers. MSC can exert their immunomodulatory effects on cells from the innate32 as well as the adaptive immune system,33,34 via direct cell–cell contact,35 in a paracrine fashion as described above, and via the release of soluble factors such as IDO, prostaglandin E2, heme-oxygenase-1, hepatocyte growth factor, transforming growth-factor-β, and IL-6.33,36 MSC in addition produce extracellular vesicles (EV) that contain a large array of cell modulatory proteins, messenger RNAs, and microRNAs (miRNA).37-39 MSC-derived EV can inhibit T, B, and natural killer (NK) cell function, possible via the shuttling of miRNA-155 and miRNA-146 into the target cells.40 The effect of EVs on these immune cells was dependent on the degree to which they were able to engulf EVs, which was most pronounced for B cells. In addition, uptake of EVs by monocytes led to the differentiation of these cells toward an M2-signature, able to enhance the function of regulatory T cells.41 Showing proof of principle, MSC-derived EVs infused into a patient with therapy-refractory GVHD led to a significant reduction of clinical symptoms.42 Taken together, MSC or derivatives thereof exhibit immunosuppressive effects, and despite all the uncertainties and unanswered questions regarding their mechanism of action, MSC are the only advanced therapy medicinal product with proven efficacy that is widely used in patients with GVHD.
T cells with immunosuppressive functions
Regulatory T cells (Treg), on the other hand, have a longstanding status as “high potential” but have not yet been able to make headway. Treg are FoxP3 and CD25 (IL-2 receptor) expressing T cells that are generated in the thymus, or in the periphery as the result of persistent stimulation of antigen experienced T cells. Together with MSC therapy, administration of ex vivo expanded Treg is the most studied form of adoptive cell therapy in the context of GVHD, as outlined very recently in a detailed review by Elias and Rudensky.43 When all published murine acute GVHD data are taken together, a picture emerges suggesting that adoptive transfer of Treg is particularly effective in a GVHD preventive setting, when co-infused with the HCT graft, and less effective when used as a therapy. In humans, adoptive transfer of ex vivo-expanded donor Tregs at day −4 successfully prevented acute GVHD in haploidentical allogeneic HCT (CD34+ stem cells plus conventional T cells) recipients that did not receive posttransplantation immunosuppression.44 Also when Tregs were co-infused with the graft in patients that did not receive additional immunosuppressive therapy posttransplantation, acute GVHD was prevented.45 These outcomes fit with earlier observations that a high Treg content of the allogeneic HCT graft is associated with a reduced risk of acute GVHD.46 In another study ex vivo expanded fourth-party cord blood-derived Tregs seemed effective in reducing acute GVHD incidence in adults receiving double cord blood-HCT, although acute GVHD incidence in the control group seemed unusually high in this study.47 Transfer of Treg together with CD34+ cells 2 days before conventional T-cell infusion in patients who had received myeloablative conditioning was feasible and not associated with excessive acute GVHD in an interim analysis of the first 12 patients.48 GVL immunity was preserved in these studies, and the rate of opportunistic infections was low because of the relatively rapid immune reconstitution observed with these regimens. There are no prospective clinical studies published demonstrating value for adoptive transfer of Tregs as therapy for acute GVHD, although a few small case series suggest potential benefit.43 See Table 1 for a list of clinical trials that are currently recruiting.
Clinical trials recruiting at the time of writing of this manuscript
| Product . | Identifier . | Cell therapy . | n . |
|---|---|---|---|
| MSC | NCT02359929 | Autologous BM-derived MSC for the treatment of acute and chronic GVHD | 24 |
| NCT02032446 | Umbilical cord derived MSC in combination with pentostatin for steroid-refractory acute GVHD | 47 | |
| NCT03847844 | Umbilical cord derived MSC for steroid-refractory acute GVHD | 40 | |
| Treg | NCT02423915 | Fucosylated Treg at day −1 pre-HCT to prevent GVHD | 47 |
| NCT01795573 | Donor Treg cells at day −2 pre-HCT to prevent GVHD | 48 | |
| NCT02749084 | Donor Treg to treat refractory chronic GVHD | 20 | |
| NCT02385019 | Donor Treg to treat refractory chronic GVHD | 22 | |
| NCT03683498 | Donor Treg to treat ruxolitinib-refractory chronic GVHD | 16 | |
| NCT01903473 | Donor Treg in combination with rapamycin to treat ruxolitinib-refractory chronic GVHD | 35 |
| Product . | Identifier . | Cell therapy . | n . |
|---|---|---|---|
| MSC | NCT02359929 | Autologous BM-derived MSC for the treatment of acute and chronic GVHD | 24 |
| NCT02032446 | Umbilical cord derived MSC in combination with pentostatin for steroid-refractory acute GVHD | 47 | |
| NCT03847844 | Umbilical cord derived MSC for steroid-refractory acute GVHD | 40 | |
| Treg | NCT02423915 | Fucosylated Treg at day −1 pre-HCT to prevent GVHD | 47 |
| NCT01795573 | Donor Treg cells at day −2 pre-HCT to prevent GVHD | 48 | |
| NCT02749084 | Donor Treg to treat refractory chronic GVHD | 20 | |
| NCT02385019 | Donor Treg to treat refractory chronic GVHD | 22 | |
| NCT03683498 | Donor Treg to treat ruxolitinib-refractory chronic GVHD | 16 | |
| NCT01903473 | Donor Treg in combination with rapamycin to treat ruxolitinib-refractory chronic GVHD | 35 |
Search terms: “graft versus host disease” and “MSC,” “Treg,” “ILC,” “dendritic cells,” “iNKT cells,” MDSC,” “CAR T cells,” and “CHAR T cells.” The latter 6 search terms did not yield any active studies.
BM, bone marrow; n, expected number of patients to be included in the trial.
One of the limitations of using conventional Tregs as adoptive therapeutic product, given the challenge to expand Treg with sufficient purity and effectivity ex vivo, is the high dose of Treg needed. Engineered Treg may offer a solution, such as HLA-A2-specific chimeric antigen receptor expressing Treg that target alloreactive T cells and prevented GVHD in mice.49 This approach has also been effective in solid organ transplant rejection and in murine models of autoimmunity,50 but has not yet been tested in humans. An even more experimental approach may be the use of virus-specific T cells engineered to express beta2-microglobulin (the universal component of HLA class 1 receptor) fused with the cytolytic endodomain of the T-cell receptor ζ chain (chimeric HLA accessory receptor [CHAR]). In a mixed lymphocyte reaction, CHAR-T cells eliminated alloreactive T cells but not pathogen-specific T cells.51 The effect of these cells on GVL immunity remains to be determined, and to the best of our knowledge these cells have not yet been tested in vivo.
Conventional Treg are not unique in their immunosuppressive competences. α/β TCR+ CD4/CD8 double negative (DN) T cells (also dubbed “DN Treg”) are an alternative subset of T cells with regulatory capacities, which can be found in low proportions (∼1%) in the blood of healthy individuals.52 DN Treg cells suppress CD4+ T-cell activity via the inhibition of mammalian target of rapamycin in effector cells.53 Adoptive transfer of ex vivo-expanded DN Treg delayed the development of GVHD in a humanized mouse model.54 Finally, adoptive transfer of ex vivo-generated rapamycin-resistant T cells 14 days after reduced-intensity allogeneic HCT, was associated with an absence of excessive GVHD in a phase 2 multicenter clinical trial including 40 patients.55 Taken together, conventional and less conventional or manufactured regulatory T cells seem to be most promising in the prevention of acute GVHD, rather than as a therapeutic product.
Myeloid derived suppressor cells
Because cells of the myeloid lineage form our first line of defense against microbes, they may not directly be associated with suppression of immune responses. Yet, myeloid-derived suppressor cells (MDSCs) appear during conditions of chronic inflammation and stress, including cancer, and then convert immunosuppressive activities.56 MDSCs can be derived from monocytic cells (M-MDSC; HLA-DR−/low CD11b+ CD14+) or granulocytic cells (or polymorphonuclear-MDSC; CD11b+ CD15+ CD33+) and are able to suppress CD3/CD28-stimulated T cells ex vivo. In the absence of a distinguishing phenotype, this functional definition is of importance, but inconvenient when evaluating patient samples.57
The first indication that MDSC could play a role in the prevention of GVHD came from an observation in a murine GVHD model, where a dramatic expansion of MDSC (from the knock-out of 5′ inositol phosphatase; SHIP−/− mice) was associated with tolerance to allogeneic T cells.58 Several studies subsequently demonstrated that the number of MDSC in allogeneic HCT grafts correlated with GVHD-free survival in mice and men,59-61 and adoptively transferred MDSC suppressed (but not abrogated) murine acute GVHD.62 Ex vivo-generated MDSC suppressed T-cell responses by depleting l-arginine, via the IL-13 induced upregulation of MDSC l-arginase expression. These results were confirmed and extended by showing that adoptive transfer of MDSC led to a skewing of T cells toward a T-helper 2 (Th2) response and the induction of Treg.63-65 Importantly, under persistent pro-inflammatory conditions (eg, when GVHD is reduced but not abrogated) inflammasome exposure leads to differentiation of MDSC away from the immune suppressive cells they were at the moment of infusion, which could be overcome by repeated MDCS transfusion.66,67 In these studies MDSC were derived from murine bone marrow; other groups demonstrated the efficacy of human cord blood derived MDSC in murine models of acute GVHD.68,69
Interestingly, MDSC expanded in the first weeks after murine allogeneic HCT, the extent of which correlated with the severity of GVHD.63 In humans, expansion of M-MDSC occurred predominantly in patients with mucositis, suggesting that M-MDSC expansion is a response to pro-inflammatory conditions.70 M-MDSC in this study produced matrix metallopeptidase 9, and levels of M-MDSC and matrix metallopeptidase 9 were associated with increased infection rates and worse outcome. In another study, higher frequencies of M-MDSC early after allogeneic HCT was associated with less GVHD but more relapse.71
Altogether, these data demonstrate that MDSC have potential as cellular therapy to prevent acute GVHD, but they are easily overstimulated leading to transition away from their suppressive function (at least in models of murine acute GVHD, the relevance of which for the human situation can be debated), or can become too immunosuppressive, abrogating GVL immune responses and leading to disease relapse. Thus, striking the right balance is a challenge with MDSC, perhaps offering an explanation why at the moment of writing of this manuscript no adoptive MDSC transfer trials are registered.
Invariant NK T cells
Invariant NK T (iNKT) cells are CD4+ or CD4− cells with an invariant α/β TCR that, when selectively activated by glycolipid antigens presented by the nonpolymorphic MHC class 1-like molecule CD1d, have immunoregulatory properties. They promote tolerance via the production of Th2 cytokines such as IL-4 and IL-13. Human allogeneic HCT recipients with relatively high frequencies of CD4− iNKT cell containing grafts had a reduced risk to develop GVHD compared with patients receiving lower proportions of CD4− iNKT cells in the graft.72 In mice, adoptive transfer of CD4+ iNKT cells at the day of allogeneic HCT protected against acute GVHD in a dose-dependent manner.73 Interestingly, these effects occurred despite the disappearance of adoptively transferred iNKT cells as early as 10 days after allogeneic HCT.74 Although iNKT cell transfer has not been demonstrated to be able to abolish acute GVHD, it did abrogate chronic GVHD in a murine model.75 Human iNKT cells isolated from peripheral blood-derived mononuclear cells can be expanded ex vivo by culturing them in the presence of agonist glycolipids,76,77 but no reports are available describing the effects of iNKT transfer in human allogeneic HCT.
Cellular therapy to prevent tissue damage and enhance tissue repair
From the studies described here in murine GVHD models and patients, it can be derived that immunomodulatory therapies are most effective when initiated during the early stages of GVHD, before overt inflammation is established and the vicious circle of inflammation and tissue damage can no longer be brought under control. An alternative approach may be to prevent tissue damage, or to stimulate swift repair of damaged tissues to prevent subsequent immune activation and alloreactivity. In fact, the association of reduced-intensity conditioning regimens with a reduced incidence of acute GVHD relies on the reduction in tissue damage.
ILC
The characteristic trait of ILC as key regulators of tissue homeostasis and repair78 qualifies ILC as candidates for adoptive therapy in the context of GVHD. Lineage (lin)− CD127+ CD117+ NKp44− and NKp44+ ILC3 can be stimulated via IL-23 to produce IL-22, which interacts with epithelial stem cells in intestinal crypts and therefore is an important contributor to gut epithelial homeostasis.79 In murine models of acute GVHD, lack of IL-22 producing ILC3 aggravated GVHD via the uncompensated destruction of intestinal stem cells by alloreactive T cells.8,80 Cotransfusion of lin− CD127+ CRTH2+ ILC2 at the time of allogeneic HCT prevented acute GVHD via the activation of MDSC.81 This GVHD protective effect was abrogated when IL-13−/− or amphiregulin−/− ILC2 were infused. Importantly, adoptive transfer of ILC2 into mice with early established acute GVHD (on day +7) also significantly improved survival, suggesting that ILC2 can be used not only to prevent acute GVHD, but also to treat acute murine GVHD.81
In humans, ILC are depleted following acute myeloid leukemia induction chemotherapy, and ILC reconstitution varied considerably between patients.12 Following allogeneic HCT, recovery of ILC was also often slow, in particular of ILC2. Delayed ILC recovery was associated with the development of GVHD, such that patients with low numbers of circulating ILC as early as 6 weeks after allogeneic HCT, at a time when these patients did not have GVHD, had an increased risk to develop acute GVHD in the weeks ensuing.12 Assuming that delayed ILC reconstitution in the blood is a reflection of impaired reconstitution of ILC in tissues, these observations suggest that delayed recovery of tissue-protective ILC in tissues precedes acute GVHD.10 Of note, and fitting with this hypothesis, we also observed that patients with lower than average ILC reconstitution after remission-induction chemotherapy and low ILC numbers at the time of allogeneic HCT had a significantly higher risk to develop mucositis and acute GVHD after allogeneic HCT.12 Taken together, these data support the notion that ILC are necessary for healthy tissue homeostasis, and that enhancement of ILC reconstitution, for example, via adoptive transfer of ILC as demonstrated in mice, may be a rational approach.
Interaction between cells
Importantly, none of the cells described here act alone (Figure 2). For example, MSC suppress activated alloreactive T cells, stimulate type 2 monocytes and macrophages that enhance Treg function, and enhance ILC proliferation and IL-22 production.30 ILC themselves not only enhance tissue repair, but in addition have direct and indirect immunosuppressive properties. We demonstrated that human ILC express the ecto-enzyme receptors CD39 and CD73, that can convert extracellular ATP (which is released upon tissue damage and acts as a DAMP in the context of GVHD) into adenosine that has immunosuppressive capacities. Ecto-enzyme expressing ILC suppressed activated T cells ex vivo, and patients with acute GVHD of the gut have reduced proportions of these ecto-enzyme expressing ILC in inflamed tissues.10 In addition, adoptively transferred ILC2 led to MDSC-induced skewing of T-cell responses toward a type 2 response and stimulation of the induction of Tregs.81 MDSC are also activated by iNKT cells, as described previously.74 Thus, the success of adoptive transfer of cells to prevent or treat acute GVHD is likely to depend on the presence of other cell types and may be enhanced when combined with other cells.
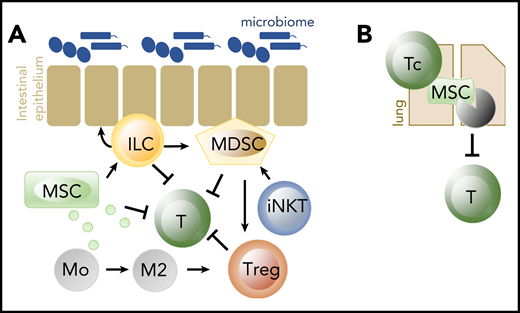
Cells important in tissue homeostasis and repair, and in suppression of alloreactivity. (A) MSC, ILC, MDSC, iNKT, and Treg have all been shown effective in the prevention of acute GVHD when adoptively transferred. Early acute GVHD could in addition be successfully treated with the adoptive transfer of ILC2. (B) Cytotoxic T cells (Tc) induce apoptosis of MSC in the lungs, which leads to suppression of adaptive lymphocytes via IDO produced by phagocytosing cells. Of all cell products, MSC are the only ones that are frequently used in human GVHD. IDO, indoleamine 2,3-dioxygenase.
Cells important in tissue homeostasis and repair, and in suppression of alloreactivity. (A) MSC, ILC, MDSC, iNKT, and Treg have all been shown effective in the prevention of acute GVHD when adoptively transferred. Early acute GVHD could in addition be successfully treated with the adoptive transfer of ILC2. (B) Cytotoxic T cells (Tc) induce apoptosis of MSC in the lungs, which leads to suppression of adaptive lymphocytes via IDO produced by phagocytosing cells. Of all cell products, MSC are the only ones that are frequently used in human GVHD. IDO, indoleamine 2,3-dioxygenase.
Conclusion
A number of cell types have been identified that have the potential to be developed as adoptive transfer product to prevent or treat acute and chronic GVHD. Most of these cell products have demonstrated potential when transferred at the time of allogeneic HCT, rather than when used as a therapy for established GVHD. This may be related to the models used. Most murine acute GVHD models represent hyperacute grade 4 GVHD that is rapidly lethal when not treated appropriately. It is possible that a single transfer of immunosuppressive or tissue reparative cells may have an underwhelming effect because of the overwhelming damage and inflammation present at the time of transfusion. This is in line with the observation that in murine models of chronic GVHD that are characterized by less massive inflammation, adoptively transferred Tregs and iNKT cells could push the immune system toward a more tolerogenic state that was associated with resolution of GVHD.75,82 Perhaps multiple cell infusions can overcome this issue, but a major hurdle will be to generate sufficient numbers of cells. Nevertheless, a single dose transfer of ILC2 (mice)81 and MSC (human)29 was found to be curative for acute GVHD, suggesting that some cell types are perhaps more effective than others. For many of the GVHD-modulating cells described in this review, it was demonstrated that having high proportions of these cells in the graft associated with less GVHD. Thus, pre-emptive adoptive transfer of GVHD-modulating cell products, at or shortly before or after the time of allogeneic HCT is probably a more feasible and effective approach.
Cell dose, frequency, and timing of adoptive transfer are probably not the only determinants of the efficacy of adoptive cell therapy. Local circumstances in recipient tissues may to a great extent determine outcome of adoptive cell transfer. This includes, for example, the presence of other immune regulatory cells such as MDSC or ILC in recipient tissues. Another determinant is probably the microbiome. In mice, it has been demonstrated that damage to the microbiome impairs hematopoiesis,83 and that microbial products such as polysaccharide A produced by the gut commensal Bacteroides fragilis can induce Tregs.84 In human allogeneic HCT recipients, antibiotic-induced loss of fecal microbiota diversity was associated with delayed recovery of M-MDSC and the occurrence of acute GVHD.85 It can therefore be hypothesized that the lifespan and function of adoptively transferred cells is affected by (the absence of) local nutrients and activating factors derived from the microbiome.
The impact of adoptive cell transfer on GVL activity is a point of concern. Adoptive transfer of ILC2 did not affect GVL reactivity, whereas in the same mouse model the transfer of Tregs in numbers high enough to prevent GVHD abrogated the GVL effect.81 In human HCT recipients, more than average MDSC expansion after allogeneic HCT has been associated with relapse.71 These data warrant caution when adoptively transferring cells with immunosuppressive effects and advocate the use of cells that primarily have tissue regeneration supportive capacities.
Taken together, adoptive transfer of cells or cellular products that manipulate either alloreactive donor cells or enhance and promote tissue homeostasis and repair may offer promise for the future and prognosis of allogeneic HCT recipients. The delicate balance between GVL and GVHD responses, and the importance to maintain GVL responses, validates a preferential focus on adoptive transfer of tissue regenerative cells rather than adoptive cell products with immunosuppressive properties.
Authorship
Contribution: C.V. and M.D.H. both performed the literature search and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mette D. Hazenberg, Amsterdam University Medical Centers, Meibergdreef 9, Amsterdam 1105 AZ, The Netherlands; e-mail: m.d.hazenberg@amc.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal