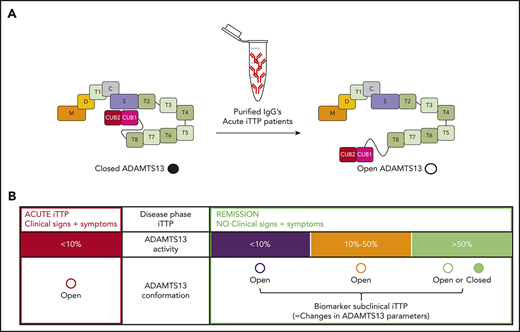
Key Points
Anti-ADAMTS13 autoantibodies from iTTP patients induce an open ADAMTS13 conformation.
An open ADAMTS13 conformation is a novel and sensitive biomarker for subclinical iTTP.
Abstract
Recently, we showed that ADAMTS13 circulates in an open conformation during the acute phase of immune-mediated thrombotic thrombocytopenic purpura (iTTP). Although the cause of this conformational change remains elusive, ADAMTS13 is primarily closed in iTTP patients in remission with ADAMTS13 activity >50% and undetectable anti-ADAMTS13 autoantibodies, as well as after rituximab treatment, suggesting a role for anti-ADAMTS13 autoantibodies. Therefore, immunoglobulin G from 18 acute iTTP patients was purified and added to closed ADAMTS13 in healthy donor plasma. This resulted in open ADAMTS13 in 14 of 18 (78%) samples, proving that anti-ADAMTS13 autoantibodies can induce an open ADAMTS13 conformation. To further elucidate the conformation of ADAMTS13 in iTTP patients, we studied a novel iTTP patient cohort (n = 197) that also included plasma samples from iTTP patients in remission in whom ADAMTS13 activity was <50%. The open ADAMTS13 conformation was found during acute iTTP, as well as in patients in remission with ADAMTS13 activity <50% and in half of the patients with ADAMTS13 activity >50%, although free anti-ADAMTS13 autoantibodies were not always detected. Thus, open ADAMTS13 is a hallmark of acute iTTP, as well as a novel biomarker that can be used to detect subclinical iTTP in patients in remission. Finally, a long-term follow-up study in 1 iTTP patient showed that the open conformation precedes a substantial drop in ADAMTS13 activity. In conclusion, we have shown that anti-ADAMTS13 autoantibodies from iTTP patients induce an open ADAMTS13 conformation. Most importantly, an open ADAMTS13 conformation is a biomarker for subclinical iTTP and could become an important tool in TTP management.
Introduction
A severe deficiency in the enzyme ADAMTS13 induced by anti-ADAMTS13 autoantibodies causes the rare and life-threatening disorder: immune-mediated thrombotic thrombocytopenic purpura (iTTP).1,2 Those anti-ADAMTS13 autoantibodies inhibit ADAMTS13 activity and/or clear ADAMTS13 from the circulation.1-5 Deficiency in ADAMTS13 results in accumulation of ultralarge von Willebrand factor (VWF) multimers, which spontaneously bind to platelets and lead to formation of microthrombi that obstruct the microvasculature. As a consequence, patients suffer from microangiopathic hemolytic anemia and severe thrombocytopenia.6-8
ADAMTS13 is a multidomain enzyme consisting of a metalloprotease domain, a disintegrin-like domain, a first thrombospondin type-1 repeat, a cysteine-rich domain, a spacer domain, 7 additional thrombospondin type-1 repeats, and 2 CUB domains.9 Under normal circumstances, ADAMTS13 adopts a closed conformation in which the spacer domain interacts with the C-terminal CUB domains.10-12 Binding of the VWF D4-CK fragment or activating murine antibodies to ADAMTS13 uncouples the spacer-CUB interaction, resulting in an open ADAMTS13 conformation.10,11,13
We generated a murine anti-ADAMTS13 monoclonal antibody (1C4) that allowed us to distinguish between the open and closed conformation of ADAMTS13.14 Antibody 1C4 recognizes an epitope in the ADAMTS13 spacer domain that is cryptic in the closed conformation but accessible in the open conformation. Using this unique tool, we recently observed that ADAMTS13 circulates in an open conformation during acute iTTP, when ADAMTS13 activity is <10% and anti-ADAMTS13 autoantibodies are detectable, whereas the conformation of ADAMTS13 is closed in the majority of iTTP patients in remission (78%), with restored ADAMTS13 activity (>50%) and undetectable anti-ADAMTS13 autoantibodies.14 Hence, we suggested open ADAMTS13 as a novel biomarker for acute iTTP. The cause of this conformational change in ADAMTS13 in acute iTTP is unknown, but anti-ADAMTS13 autoantibodies could play a role, because ADAMTS13 conformation is closed in iTTP patients with restored ADAMTS13 activity (>50%) after rituximab treatment.15
ADAMTS13 activity <10% is a unique and well-established biomarker to diagnose TTP. During remission, changes in ADAMTS13 parameters (ADAMTS13 activity, antigen, and anti-ADAMTS13 autoantibodies) can occur,16-18 which we define here as subclinical disease. Indeed, patients in remission with ADAMTS13 activity <10% or anti-ADAMTS13 autoantibodies have a higher risk for clinical relapse and, therefore, are often (re)treated with rituximab15-17,19-26 ; however, monitoring and management of iTTP patients during remission are less well established. It is unknown whether ADAMTS13 conformation is a novel biomarker for subclinical disease that could further guide the management of iTTP patients during the remission phase.
In this study, we investigated whether anti-ADAMTS13 autoantibodies from acute iTTP patients induce conformational changes in ADAMTS13. We also investigated whether, in addition to ADAMTS13 activity, ADAMTS13 conformation could be used as a biomarker to detect subclinical iTTP, by analyzing ADAMTS13 conformation in a new multicenter cohort of iTTP patients during acute and remission phases. Finally, to assess whether ADAMTS13 conformation can be used as a biomarker to follow disease progression and detect the early stages of subclinical iTTP, ADAMTS13 conformation was measured as a function of time in an individual iTTP patient.
Patients, materials, and methods
Mouse anti-human ADAMTS13 antibodies
Several monoclonal mouse anti-human ADAMTS13 antibodies that were developed in-house were used (see supplemental Patients, materials, and methods, available on the Blood Web site).
iTTP patient plasma samples
iTTP plasma samples taken in the acute or remission phase were available from the TTP centers in Marseille, Milan, Budapest, Utrecht, Mainz, and Paris (supplemental Tables 1-3). From the 19 acute iTTP patients from the center in Marseille (supplemental Table 1), large plasma volumes from their first plasmapheresis bags were available and used to purify immunoglobulin G (IgG) (see "Purification of the IgG antibody fraction from plasma"). A total of 209 citrated plasma samples from 140 iTTP patients were available from the centers in Milan, Budapest, Utrecht, and Mainz (supplemental Table 2) to study the ADAMTS13 conformation in acute and remission phases. In addition, 39 consecutive samples from 1 iTTP patient from the TTP reference center in Paris were available that allowed us to assess conformational changes in ADAMTS13 as a function of time starting from the first acute episode (supplemental Table 3). ADAMTS13 conformation in 15 of these 39 samples has been reported previously.15
Informed consent was obtained from each patient according to the Declaration of Helsinki, and the study was approved by the Ethics Committees of Projet National de Recherche Clinique 2007 (Marseille, France), Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Human Clinical Research (Budapest, Hungary), the University Medical Center Utrecht, Ethik-Kommission, Landesärztekammer Rheinland-Pfalz (Mainz, Germany), Hôpital Pitié-Salpêtrière (Paris, France), and Hôpital Saint-Antoine for their respective samples.
Acute iTTP was defined as a thrombotic microangiopathic syndrome (microangiopathic hemolytic anemia with thrombocytopenia, with or without organ involvement) associated with severe ADAMTS13 deficiency (activity <10%), whereas iTTP in remission was defined as a sustained normalization of platelet count and lactate dehydrogenase levels for >30 days after cessation of plasma exchange.27
Healthy donor plasma samples
Plasma from nonremunerated healthy blood donors was obtained from the Blood Service of the Belgian Red Cross-Flanders. Citrated plasma from 10 healthy donors (supplemental Table 4) was used as controls in Figure 1. Additionally, plasma from 404 healthy donors was used to determine the cutoff of our anti-ADAMTS13 autoantibody enzyme-linked immunosorbent assay (ELISA) (see "Anti-ADAMTS13 autoantibody ELISA"). Informed consent for purposes of scientific research was obtained from each healthy donor according to the Declaration of Helsinki, and the study was approved by the Ethics Committee of KU Leuven.
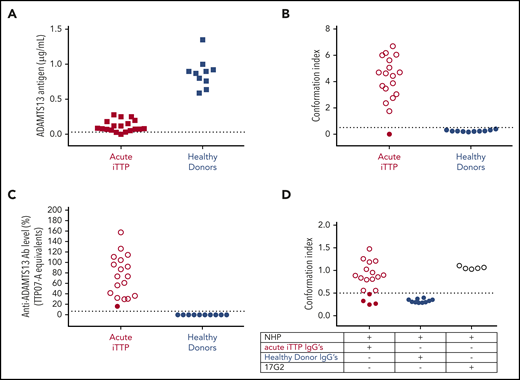
Anti-ADAMTS13 autoantibodies from acute iTTP patients induce an open ADAMTS13 conformation in healthy donor plasma. (A) ADAMTS13 antigen levels in acute iTTP patients (n = 19) and healthy donors (n = 10). Plasma was added to coated antibody 3H9. Bound ADAMTS13 was detected using biotinylated antibodies 19H4 and 17G2 and horseradish peroxidase–labeled streptavidin. NHP was used as a reference and set as 1 µg/mL ADAMTS13.5 Only samples containing ≥0.03 µg/mL (dotted line) could be tested for ADAMTS13 conformation. (B) ADAMTS13 conformation was tested in plasma samples of acute iTTP patients (n = 18) and healthy donors (n = 10) using our 1C4-ELISA; open ADAMTS13 (Conformation Index >0.5; open circles), but not closed ADAMTS13 (Conformation Index ≤0.5; filled circles), is specifically captured on the coated antibody 1C4. (C) Anti-ADAMTS13 autoantibody levels in acute iTTP patients (n = 18) and healthy donors (n = 10). Plasma was added to coated rhADAMTS13, and bound anti-ADAMTS13 autoantibodies were detected by horseradish peroxidase–labeled polyclonal goat anti-human IgG (Fc-specific) antibodies. To calculate anti-ADAMTS13 autoantibody levels, the iTTP plasma sample TTP07-A was used as a reference and set as 100%. The threshold of positivity was determined by the 97.5th percentile of anti-ADAMTS13 autoantibody levels measured in healthy donors (n = 404) and set as 6.7% (supplemental Figure 1). Filled circles, closed ADAMTS13; open circles, open ADAMTS13. (D) Purified IgGs from acute iTTP patients (n = 18) or healthy donors (n = 10) were individually preincubated with closed ADAMTS13 present in NHP. Next, the preincubated samples were tested using the 1C4-ELISA to determine whether the purified IgGs had opened the closed ADAMTS13 conformation in NHP. Murine monoclonal antibody 17G2, which induces an open ADAMTS13 conformation, was used as a positive control (n = 5). Filled circles, closed ADAMTS13; open circles, open ADAMTS13.
Anti-ADAMTS13 autoantibodies from acute iTTP patients induce an open ADAMTS13 conformation in healthy donor plasma. (A) ADAMTS13 antigen levels in acute iTTP patients (n = 19) and healthy donors (n = 10). Plasma was added to coated antibody 3H9. Bound ADAMTS13 was detected using biotinylated antibodies 19H4 and 17G2 and horseradish peroxidase–labeled streptavidin. NHP was used as a reference and set as 1 µg/mL ADAMTS13.5 Only samples containing ≥0.03 µg/mL (dotted line) could be tested for ADAMTS13 conformation. (B) ADAMTS13 conformation was tested in plasma samples of acute iTTP patients (n = 18) and healthy donors (n = 10) using our 1C4-ELISA; open ADAMTS13 (Conformation Index >0.5; open circles), but not closed ADAMTS13 (Conformation Index ≤0.5; filled circles), is specifically captured on the coated antibody 1C4. (C) Anti-ADAMTS13 autoantibody levels in acute iTTP patients (n = 18) and healthy donors (n = 10). Plasma was added to coated rhADAMTS13, and bound anti-ADAMTS13 autoantibodies were detected by horseradish peroxidase–labeled polyclonal goat anti-human IgG (Fc-specific) antibodies. To calculate anti-ADAMTS13 autoantibody levels, the iTTP plasma sample TTP07-A was used as a reference and set as 100%. The threshold of positivity was determined by the 97.5th percentile of anti-ADAMTS13 autoantibody levels measured in healthy donors (n = 404) and set as 6.7% (supplemental Figure 1). Filled circles, closed ADAMTS13; open circles, open ADAMTS13. (D) Purified IgGs from acute iTTP patients (n = 18) or healthy donors (n = 10) were individually preincubated with closed ADAMTS13 present in NHP. Next, the preincubated samples were tested using the 1C4-ELISA to determine whether the purified IgGs had opened the closed ADAMTS13 conformation in NHP. Murine monoclonal antibody 17G2, which induces an open ADAMTS13 conformation, was used as a positive control (n = 5). Filled circles, closed ADAMTS13; open circles, open ADAMTS13.
ADAMTS13 activity
ADAMTS13 activity levels were measured, using a FRETS-VWF73 assay,28,29 in all iTTP and healthy donor plasma samples (supplemental Tables 1-4). A serial dilution of a healthy donor plasma pool (NHP) containing citrated plasma of 20 healthy donors (set as 100% ADAMTS13 activity) was used to calculate ADAMTS13 activities.
ADAMTS13 antigen ELISA
ADAMTS13 antigen levels in the 267 iTTP plasma samples and 10 healthy donors (supplemental Tables 1-4) were determined by ELISA, as previously described (see supplemental Patients, materials, and methods for details).5,14 NHP was used as a reference and set as 1 µg/mL (detection limit, 0.02 µg/mL).5,14
ADAMTS13 conformation ELISA
ADAMTS13 conformation was determined using the previously described ADAMTS13 conformation ELISA (1C4-ELISA).14 ADAMTS13 conformation could be determined in 254 iTTP plasma samples and in 10 healthy donor plasma samples (ADAMTS13 antigen ≥0.03 µg/mL, the detection limit for the 1C4-ELISA).14 In brief, a 96-well microtiter plate was coated with the antibody 1C4 (5 µg/mL). After blocking, 1:4 diluted plasma from healthy donors or iTTP patients was added. After washing, addition of biotinylated antibody 3H9 (1.5 µg/mL) and horseradish peroxidase–labeled high-sensitivity streptavidin (1:10 000; Invitrogen, Carlsbad, CA) was used to detect bound open ADAMTS13. O-phenylenediamine and H2O2 were used to initiate the colorimetric reaction, which was stopped by addition of 4 M sulfuric acid. As previously described,14 a twofold dilution series of NHP (starting dilution 1:2; 0.5 µg/mL ADAMTS13) preincubated with 2.5 µg/mL anti-CUB1 antibody 17G2,13 which induces an open ADAMTS13 conformation, was added as a reference curve and intra-assay control. To calculate conformation indices, the obtained optical density at 490 nm (OD490nm) values were first corrected for ADAMTS13 antigen. Next, the antigen-corrected OD490nm values were normalized using the OD490nm value of the intra-assay control (NHP [diluted 1:4] in the presence of antibody 17G2). A conformation index ≤0.5 represents closed ADAMTS13, whereas a conformation index >0.5 represents open ADAMTS13.14
Anti-ADAMTS13 autoantibody ELISA
The anti-ADAMTS13 autoantibody levels in plasma samples were determined using ELISA. To determine the cutoff value for positivity of our anti-ADAMTS13 autoantibody ELISA, microtiter plates were coated with recombinant human ADAMTS13 (rhADAMTS13; 15 nM). After blocking, plasma from each of the 404 healthy donors (each diluted 1:40) was added. Bound anti-ADAMTS13 autoantibodies were detected using horseradish peroxidase–labeled polyclonal goat anti-human IgG (Fc-specific) antibodies (1:10 000; Sigma-Aldrich, St. Louis, MO). The colorimetric reaction was performed as described above. Sample TTP07-A (Marseille cohort), with a high anti-ADAMTS13 autoantibody level, was set as 100% and used as a reference curve to calculate anti-ADAMTS13 autoantibody levels in TTP07-A equivalents (%). The 97.5th percentile of the 404 healthy donor anti-ADAMTS13 autoantibody levels was determined and set as the cutoff for positivity for anti-ADAMTS13 autoantibodies. Next, the anti-ADAMTS13 autoantibody levels in the 254 iTTP plasma samples and in the 10 healthy donor plasma samples (start dilution, 1:20) were determined, as described above.
Purification of the IgG antibody fraction from plasma
Total IgG antibodies were purified from 18 acute iTTP plasma samples in the cohort from Marseille (supplemental Table 1) and in 10 healthy donors (supplemental Table 4), using Protein G Sepharose Fast Flow (GE Healthcare, Waukesha, WI) affinity chromatography, as previously described.13 IgG concentrations were determined via spectrophotometry at OD280nm, and purity was checked using sodium dodecyl sulfate polyacrylamide gel electrophoresis, followed by Instant Blue Protein Gel staining (Westburg; Leusden, The Netherlands). Purified total IgGs (200 µg/mL) were tested for their binding to rhADAMTS13 using the anti-ADAMTS13 autoantibody ELISA, as described above.
1C4-ELISA to detect whether purified iTTP IgGs induce open ADAMTS13 in healthy donor plasma
To determine whether the anti-ADAMTS13 autoantibodies from iTTP patients induce an open ADAMTS13 conformation, individual purified IgG fractions from the 18 acute iTTP patients (concentration range, 449-1372 µg/mL; diluted 1:2) were added to closed ADAMTS13 present in NHP (diluted 1:4), and the ADAMTS13 conformation was tested using the 1C4-ELISA, as described above.
Statistics
The Fisher’s exact test was performed using GraphPad Prism 6 software (GraphPad Software, San Diego, CA). P < .05 was considered significant.
Results
Anti-ADAMTS13 autoantibodies induce the open ADAMTS13 conformation
Before investigating whether anti-ADAMTS13 autoantibodies could be responsible for the conformational change in ADAMTS13 observed during acute iTTP, we needed to know which acute iTTP patients had an open ADAMTS13 conformation and detectable anti-ADAMTS13 autoantibodies. Therefore, we first determined ADAMTS13 antigen, conformation, and anti-ADAMTS13 autoantibody levels in plasma from 19 acute iTTP patients (Marseille cohort; supplemental Table 1) and 10 healthy donors (supplemental Table 4). ADAMTS13 antigen levels were needed, because ADAMTS13 conformation can only be assessed with ADAMTS13 antigen levels ≥0.03 μg/mL.14 As expected, ADAMTS13 antigen levels in the 19 acute iTTP patients were reduced (range, <0.02-0.28 µg/mL) compared with the levels observed in the 10 healthy donors (range, 0.59-1.35 µg/mL) (Figure 1A). Because 1 acute iTTP sample did not contain sufficient ADAMTS13 antigen (TTP07-A; supplemental Table 1), it was excluded from further analysis. ADAMTS13 conformation was open in 17 acute iTTP samples, whereas it was closed in 1 acute iTTP patient and all 10 healthy donor samples (Figure 1B), which confirms our previous results.14 All 18 acute iTTP patient samples, in which ADAMTS13 conformation could be determined, had detectable anti-ADAMTS13 autoantibody levels, whereas the healthy donors were negative for anti-ADAMTS13 autoantibodies (Figure 1C; threshold for anti-ADAMTS13 autoantibody positivity was 6.7% and had been determined using 404 healthy donor plasma samples, supplemental Figure 1).
Hence, to test the potential involvement of anti-ADAMTS13 autoantibodies in the conformational change in ADAMTS13 in acute iTTP patients, total IgGs from the 18 acute iTTP plasma samples, as well as from the 10 healthy donors, were isolated. The isolated IgGs from the 18 iTTP patients still bound to coated rhADAMTS13 in ELISA, in contrast to the isolated control IgGs from healthy donors (data not shown). Next, the purified IgGs were preincubated with NHP, containing closed ADAMTS13, and tested using 1C4-ELISA to assess whether the purified IgGs induce the open ADAMTS13 conformation. Intriguingly, the purified IgGs of 14 of the 17 (82%) acute iTTP patients with an open conformation changed the conformation of closed NHP ADAMTS13 to an open one (Figure 1D). This unequivocally shows that anti-ADAMTS13 autoantibodies from acute iTTP patients are indeed able to change the ADAMTS13 conformation. As expected, the purified IgGs from the acute iTTP patient with a closed conformation and from all healthy donors could not open ADAMTS13 in NHP (Figure 1D). Finally, antibody 17G2 was included as a positive control and induced an open ADAMTS13 conformation in NHP, as expected (Figure 1D).
In summary, we demonstrated that anti-ADAMTS13 autoantibodies isolated from plasma of acute iTTP patients with an open ADAMTS13 conformation can induce a conformational change in ADAMTS13 of NHP from a closed form to an open form in the majority of cases.
Open ADAMTS13 conformation is a novel biomarker for subclinical iTTP
We previously reported that the conformation of ADAMTS13 is open in acute iTTP patients and closed in the majority of iTTP patients in remission with restored ADAMTS13 activity (>50%).14 However, iTTP patients in remission are at risk for relapse, when ADAMTS13 activity is decreased or anti-ADAMTS13 autoantibodies are present,17 indicating an ongoing subclinical disease (defined as changes in ADAMTS13 parameters during remission). To investigate whether, in addition to ADAMTS13 activity, ADAMTS13 conformation is a biomarker to identify subclinical iTTP in patients in remission, we determined the ADAMTS13 conformation in plasma in a new multicenter iTTP patient cohort, including samples taken during acute (ADAMTS13 activity <10%) and remission phases with restored (>50%), partly restored (10-50%), or completely deficient (<10%) ADAMTS13 activity. In total, 140 iTTP patients were included, for which 209 plasma samples were available (Figure 2A; supplemental Table 2). From these 209 iTTP plasma samples, 41 samples from 41 different iTTP patients were obtained during an acute iTTP episode (Figure 2A; supplemental Table 2). The other 168 samples were retrieved from all 140 iTTP patients during remission. ADAMTS13 activity levels were used to divide samples into 4 groups: acute iTTP (ADAMTS13 activity <10%; n = 41) and iTTP in remission (ADAMTS13 activity <10%, n = 45; ADAMTS13 activity, 10-50% (n = 32); and ADAMTS13 activity >50%, n = 91) (Figure 2A; supplemental Table 2).
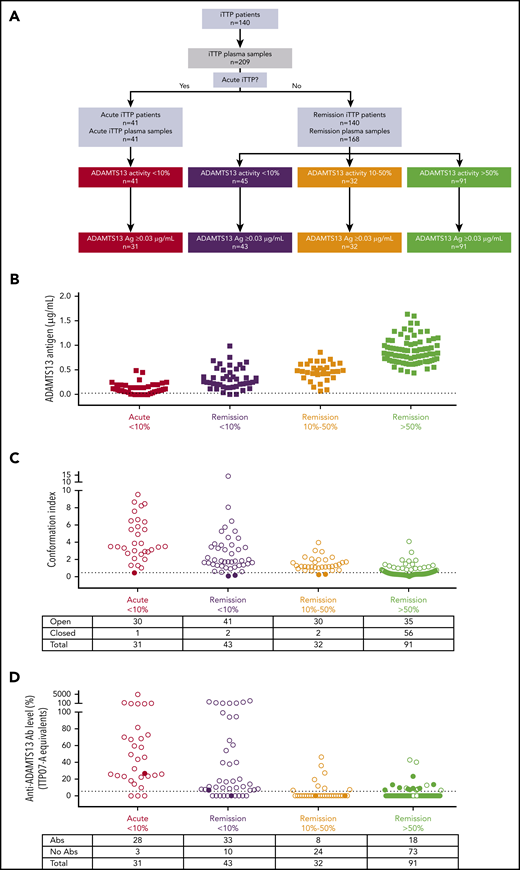
ADAMTS13 antigen levels, ADAMTS13 conformation, and anti-ADAMTS13 autoantibody levels in iTTP patient samples during acute and remission phase. (A) Flow chart of patient inclusion. iTTP plasma samples (n = 209) from 140 iTTP patients were grouped into 4 categories according to their ADAMTS13 activity and disease state: acute iTTP (ADAMTS13 activity <10%, n = 41), iTTP in remission with ADAMTS13 activity <10% (n = 45), iTTP in remission with ADAMTS13 activity between 10% and 50% (n = 32), and iTTP in remission with ADAMTS13 activity >50% (n = 91). (B) ADAMTS13 antigen levels in plasma from acute iTTP patients (n = 41) and iTTP patients in remission (n = 140).5 Only samples containing ≥0.03 µg/mL (dotted line) could be tested for ADAMTS13 conformation. (C) ADAMTS13 conformation was tested in plasma samples from acute iTTP patients (n = 31), iTTP patients in remission with ADAMTS13 activity <10% (n = 43), iTTP patients in remission with ADAMTS13 activity between 10% and 50% (n = 32), and iTTP patients in remission with ADAMTS13 activity >50% (n = 91), using 1C4-ELISA. Filled circles, closed ADAMTS13; open circles, open ADAMTS13. (D) Anti-ADAMTS13 autoantibody levels (TTP07-A equivalents) in plasma from the patients in C were measured using ELISA. Filled circles, closed ADAMTS13; open circles, open ADAMTS13. Abs, antibodies.
ADAMTS13 antigen levels, ADAMTS13 conformation, and anti-ADAMTS13 autoantibody levels in iTTP patient samples during acute and remission phase. (A) Flow chart of patient inclusion. iTTP plasma samples (n = 209) from 140 iTTP patients were grouped into 4 categories according to their ADAMTS13 activity and disease state: acute iTTP (ADAMTS13 activity <10%, n = 41), iTTP in remission with ADAMTS13 activity <10% (n = 45), iTTP in remission with ADAMTS13 activity between 10% and 50% (n = 32), and iTTP in remission with ADAMTS13 activity >50% (n = 91). (B) ADAMTS13 antigen levels in plasma from acute iTTP patients (n = 41) and iTTP patients in remission (n = 140).5 Only samples containing ≥0.03 µg/mL (dotted line) could be tested for ADAMTS13 conformation. (C) ADAMTS13 conformation was tested in plasma samples from acute iTTP patients (n = 31), iTTP patients in remission with ADAMTS13 activity <10% (n = 43), iTTP patients in remission with ADAMTS13 activity between 10% and 50% (n = 32), and iTTP patients in remission with ADAMTS13 activity >50% (n = 91), using 1C4-ELISA. Filled circles, closed ADAMTS13; open circles, open ADAMTS13. (D) Anti-ADAMTS13 autoantibody levels (TTP07-A equivalents) in plasma from the patients in C were measured using ELISA. Filled circles, closed ADAMTS13; open circles, open ADAMTS13. Abs, antibodies.
Determination of ADAMTS13 antigen levels (Figure 2B) showed that, of the 209 iTTP samples, 12 had to be excluded from further conformation analysis because ADAMTS13 antigen levels were <0.03 µg/mL (Figure 2B; supplemental Table 2).14 Ten of the excluded samples were from acute iTTP patients, whereas 2 were from patients in remission with ADAMTS13 activity <10% (Figure 2A-B; supplemental Table 2).
Next, ADAMTS13 conformation was determined in the remaining 197 iTTP samples using 1C4-ELISA (Figure 2C; supplemental Table 2). In line with our previous work,14 an open ADAMTS13 conformation was found in almost all acute iTTP plasma samples (97%, 30/31), whereas it was closed in more than half of the plasma samples from patients in remission with ADAMTS13 activity >50% (62%, 56/91) (Figure 2C; supplemental Table 2). Remarkably, an open ADAMTS13 conformation was detected in almost all remission samples with ADAMTS13 activity <10% (95%, 41/43) and even in those with ADAMTS13 activity between 10% and 50% (94%, 30/32) (Figure 2C; supplemental Table 2).
Hence, these data show that there is a significant association (Fisher exact test, P < .0001; supplemental Figure 2B) between decreased ADAMTS13 activity (<50%) and an open ADAMTS13 conformation (96%, 102/106). Interestingly, however, 38% (35/91) of the iTTP plasma samples with restored ADAMTS13 activity (>50%) had an open ADAMTS13 conformation, suggesting that ADAMTS13 conformation is a more sensitive biomarker for subclinical disease than is ADAMTS13 activity.
In summary, open ADAMTS13 is observed during acute iTTP, as well as during remission, especially when ADAMTS13 activity is decreased (<50%), indicating that open ADAMTS13 is a novel biomarker to detect subclinical iTTP in remission.
Open ADAMTS13 conformation is a more sensitive biomarker to detect subclinical iTTP than the presence of anti-ADAMTS13 autoantibodies
At many centers, the disease course in iTTP patients in remission is monitored by measuring ADAMTS13 activity rather than anti-ADAMTS13 autoantibodies. Because decreased ADAMTS13 activity suggests the presence of anti-ADAMTS13 autoantibodies, the anti-ADAMTS13 autoantibody ELISA is not routinely used when patients are in remission. Hence, although it is well known that anti-ADAMTS13 autoantibodies are present in the acute phase, less information is available about anti-ADAMTS13 autoantibody titers when patients are in remission. Therefore, we measured anti-ADAMTS13 autoantibody levels in all 197 iTTP patient samples, of which 166 were from iTTP patients in remission (Figure 2D; supplemental Table 2). As expected, detectable anti-ADAMTS13 autoantibodies were present in the majority of the acute iTTP and remission samples with ADAMTS13 activity <10% (90% and 77%, respectively; Figure 2D), whereas only 20% of the remission samples with restored ADAMTS13 activity (>50%) had detectable anti-ADAMTS13 autoantibodies (Figure 2D). In the group with a moderately decreased ADAMTS13 activity (10-50%), anti-ADAMTS13 autoantibodies were detected in 25% of the samples (Figure 2D), whereas 94% (30/32) of those samples had an open ADAMTS13 conformation (Figure 2C). Hence, the presence of anti-ADAMTS13 autoantibodies and open ADAMTS13 conformation are associated (Fisher’s exact test, P <.0001; supplemental Figure 2C), and open ADAMTS13 conformation might be a surrogate and sensitive biomarker to detect anti-ADAMTS13 autoantibodies and, hence, subclinical iTTP.
Open ADAMTS13 conformation as an early biomarker to detect the onset of subclinical iTTP
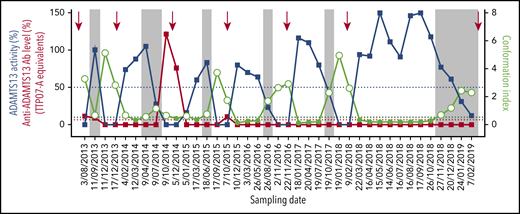
To validate the use of open ADAMTS13 conformation as a more sensitive biomarker than ADAMTS13 activity or anti-ADAMTS13 autoantibodies for subclinical iTTP, we retrospectively studied ADAMTS13 activity, conformation, and anti-ADAMTS13 autoantibody levels starting from the first acute episode and for 38 consecutive time points in a single iTTP patient (Figure 3; supplemental Table 3). During the first acute episode, the patient was treated with plasma exchanges and received rituximab. The patient was followed approximately every 3 months for the first 4 years and monthly during the last year. During follow-up, ADAMTS13 activity was measured (Figure 3, blue squares); when the activity dropped below 10%, the patient was treated with preemptive rituximab (Figure 3, red arrows). After the first acute episode, the patient experienced 6 more substantial decreases in ADAMTS13 activity (<10%) (Figure 3, blue squares).
Open ADAMTS13 conformation is a novel and early biomarker of subclinical iTTP. ADAMTS13 activity (blue squares), anti-ADAMTS13 autoantibody levels (red squares), and ADAMTS13 conformation (open circles, open ADAMTS13; filled light green circles, closed ADAMTS13) were determined in 39 consecutive plasma samples from 1 iTTP patient. Dates of sampling are indicated. The red arrows represent rituximab infusion. The vertical gray boxes highlight that ADAMTS13 conformation is open before a substantial decrease in ADAMTS13 activity (<10%). The blue dotted lines indicate 10% and 50% ADAMTS13 activity, while the red dotted line represents the cutoff for anti-ADAMTS13 autoantibody positivity (6.7%).
Open ADAMTS13 conformation is a novel and early biomarker of subclinical iTTP. ADAMTS13 activity (blue squares), anti-ADAMTS13 autoantibody levels (red squares), and ADAMTS13 conformation (open circles, open ADAMTS13; filled light green circles, closed ADAMTS13) were determined in 39 consecutive plasma samples from 1 iTTP patient. Dates of sampling are indicated. The red arrows represent rituximab infusion. The vertical gray boxes highlight that ADAMTS13 conformation is open before a substantial decrease in ADAMTS13 activity (<10%). The blue dotted lines indicate 10% and 50% ADAMTS13 activity, while the red dotted line represents the cutoff for anti-ADAMTS13 autoantibody positivity (6.7%).
When studying ADAMTS13 conformation in all samples during this 5-year follow-up study, we confirmed that an open ADAMTS13 conformation is indeed a biomarker for acute iTTP, as well as for subclinical disease in remission. ADAMTS13 conformation (Figure 3, light green open circles) was open during all 7 periods of substantially decreased ADAMTS13 activity. Most importantly, an open ADAMTS13 conformation seemed to precede the drop of ADAMTS13 activity to <10% (Figure 3, gray boxes), suggesting that, in this patient, an open ADAMTS13 conformation is an early sign of changes in ADAMTS13 in remission and, hence, is a sensitive biomarker for subclinical disease. In addition, although ADAMTS13 conformation was open, free anti-ADAMTS13 autoantibodies were detected only in a minority of the samples (Figure 3, red squares; supplemental Table 3). Most likely, the autoantibodies were already adsorbed to ADAMTS13 and cleared together; therefore, they could not bind to the antigen in the ELISA. This is consistent with the clinical observation that the patient responded transiently, each time, to rituximab, suggesting the presence of anti-ADAMTS13 autoantibodies (Figure 3). These data show that ADAMTS13 conformation is a sensitive biomarker for subclinical iTTP in remission.
Discussion
In this study, we identified that a subgroup of anti-ADAMTS13 autoantibodies (“opening” antibodies) can induce an open ADAMTS13 conformation. Additionally, we showed that an open ADAMTS13 conformation is present in acute iTTP patients, as well as in iTTP patients in clinical remission with decreased ADAMTS13 activity <50% (subclinical disease). Even iTTP patients in remission with ADAMTS13 activity >50% have an open ADAMTS13 conformation in 22%14 to 38% of cases, revealing that a change in ADAMTS13 conformation in remission is a novel and sensitive biomarker to detect the early stages of subclinical iTTP. In line with this, a long-term follow-up of 1 specific iTTP patient showed that, in this case, substantial decreases in ADAMTS13 activity (<10%) were consistently preceded by the appearance of an open ADAMTS13 conformation. However, to prove that open ADAMTS13 can predict a decrease in ADAMTS13 activity, a novel study is needed using a large cohort of iTTP patients from whom multiple consecutive plasma samples are available over a period of several years.
We have shown that 14 purified IgGs isolated from 17 individual acute iTTP patients induced conformational changes in ADAMTS13, whereas IgGs from the remaining 3 patients did not, although “opening” antibodies were expected to be present, because an open ADAMTS13 conformation was detected in the plasma of these 3 iTTP patients. It is possible that the “opening” anti-ADAMTS13 autoantibodies are in complex with ADAMTS13 and, therefore, are not available to bind and open up additional ADAMTS13. Alternatively, the concentration of these antibodies in the purified IgG fraction was lower compared with their, presumably already low, concentration in plasma and might be too low to still have an effect. The third option is that anti-ADAMTS13 autoantibodies of the IgM or IgA class, which have also been described in iTTP patients,21,23,30 are present and could be responsible for the open ADAMTS13 conformation in the plasma samples of those 3 patients.
Several studies showed that ADAMTS13 activity <10% and/or presence of anti-ADAMTS13 autoantibodies during remission indicate a higher risk for relapse.16,17,20,21,23,24,27,31 The presence of an open ADAMTS13 conformation in most of our remission patients with decreased ADAMTS13 activity further underscores their ongoing subclinical disease. Currently, if ADAMTS13 activity in iTTP patients in remission drops below 10%, preemptive rituximab treatment, which depletes proliferating and antibody-producing B cells, is initiated at many centers to prevent relapses.15,25,26 Recently, we showed that ADAMTS13 is closed after rituximab treatment in iTTP patients.15 Identification of “opening” anti-ADAMTS13 autoantibodies in most acute iTTP patients in the current study explains why rituximab treatment leads to the reappearance of closed ADAMTS13. Our findings further emphasize the importance of preemptive rituximab treatment in remission patients with substantially decreased ADAMTS13 activity (<10%),15,19,25,26 even though the effectiveness of rituximab in suppressing pathogenic anti-ADAMTS13 autoantibodies may be limited in time.25,32,33 In a long-term follow-up of 1 individual patient, we showed that preemptive rituximab helped to restore ADAMTS13 activity several times. Interestingly, restoration of ADAMTS13 activity did not always coincide with restoration from open to closed ADAMTS13, indicating an ongoing subclinical disease. Potentially, open ADAMTS13 might become a biomarker to monitor therapeutic effectiveness and follow disease progression to detect subclinical iTTP.
Our study also indicates that open ADAMTS13 might be a more sensitive biomarker for the presence of anti-ADAMTS13 autoantibodies than the current anti-ADAMTS13 autoantibody assays. Indeed, the long-term follow-up study showed that anti-ADAMTS13 autoantibodies were only detected at 5 of the 39 time points, despite decreased ADAMTS13 activity at several time points. Also, in the remission patients with decreased ADAMTS13 activity (<50%), anti-ADAMTS13 autoantibodies were observed in only 55% of the cases, although the majority of the samples had an open ADAMTS13 conformation, suggesting that ADAMTS13 conformation could be a surrogate marker to uncover undetectable anti-ADAMTS13 autoantibodies in iTTP patients. Only free anti-ADAMTS13 autoantibodies are detected with current ELISAs; however, immune complexes have been found in acute iTTP patients, as well as when they are in remission.34,35 This underscores that adsorption of autoantibodies to endogenous ADAMTS13 is a very likely reason for the negative antibody test, a phenomenon that is very well-known, for example, in red cell and platelet autoantibodies.
Previous studies have shown that anti-ADAMTS13 autoantibodies from iTTP patients can clear and/or inhibit ADAMTS13.1-5 This study identified that anti-ADAMTS13 autoantibodies can also change the conformation of ADAMTS13; however, the exact role of the “opening” anti-ADAMTS13 autoantibodies in iTTP pathophysiology and whether they are linked to disease severity is not known. Whether the “opening” anti-ADAMTS13 autoantibodies have a specific role and trigger an additional immune response as a result of the exposure of cryptic epitopes and whether they are the result of the polyclonal immune response triggered by an external factor (eg, infection, stress) need to be investigated further. The initial trigger leading to the development of anti-ADAMTS13 autoantibodies is unknown, although predisposing genetic risk factors involving the HLA system have been identified.36 Investigating whether the opening anti-ADAMTS13 autoantibodies represent a very early step in the immune response against ADAMTS13 and investigating which epitopes in ADAMTS13 are targeted by the opening autoantibodies will provide more insight into their role and show whether blocking those “opening” anti-ADAMTS13 autoantibodies (with, for example, anti-idiotypic antibodies37 or small molecules) would be a feasible new therapeutic strategy.
In summary, we found that anti-ADAMTS13 autoantibodies can induce conformational changes in ADAMTS13. We also showed that, in addition to acute iTTP patients, ADAMTS13 conformation is open in iTTP patients in remission, and an open ADAMTS13 conformation might even appear before a substantial drop in ADAMTS13 activity. Therefore, open ADAMTS13 could become a novel and sensitive biomarker to monitor iTTP patients and identify the early stages of subclinical iTTP (changes in ADAMTS13 parameters). Further work should assess whether open ADAMTS13 and “opening” anti-ADAMTS13 autoantibodies contribute to ADAMTS13 deficiency in iTTP patients and how measuring open ADAMS13 during remission could improve the management of iTTP patients during follow-up.
Data sharing requests should be sent to Karen Vanhoorelbeke (karen.vanhoorelbeke@kuleuven.be).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank N. Vandeputte and A. Dewaele for excellent technical assistance, S. Benghezal and S. Capdenat for assistance with obtaining plasma samples from the Biobank of the French Reference Center for Thrombotic MicroAngiopathies, and Sandrine F. for commitment and dedication in donating blood for the long-term follow-up study.
This work was supported by The European Framework Program for Research and Innovation (Horizon2020 Marie Sklodowska Curie Innovative Training Network PROFILE Grant 675746), Semmelweis University/KU Leuven (CELSA Research Grant 2018), KU Leuven (OT/14/071), a Projet National de Recherche Clinique 2007 (2007/23) (Marseille, France) and the Centre Constitutif des Microangiopathies Thrombotiques, Région PACA. The Mainz cohort study on iTTP patients was funded by the Bundesministerium für Bildung und Forschung. A.-S.S. is supported by a PhD grant from the Agency for Innovation and Entrepreneurship (Flanders, Belgium; 141136).
Authorship
Contribution: E.R., A.-S.S., C.D., A. Vandenbulcke, and I.P. performed experiments; E.R., A.-S.S., C.D., and K.V. designed the experiments, analyzed and interpreted the data, and wrote the manuscript; E.T., G.S., B.S.J., G.K., M.L.B., I.M., T.F., C.V.A., M.R., H.R., Y.B., R.F., Z.P., F.P., B.L., P.C., and A. Veyradier provided iTTP patient samples and critically reviewed the manuscript; H.B.F. provided healthy donor plasma samples and critically reviewed the manuscript; and J.V., A.G., H.D., and S.F.D.M. provided helpful discussions and critically reviewed the manuscript.
Conflict-of-interest disclosure: I.M. has received lecture fees by Ablynx-Sanofi and IL-Werfen. A. Veyradier is a member of the French Advisory board for caplacizumab (Ablynx-Sanofi). B.L. is on the Advisory Board for Albynx-Sanofi for caplacizumab, holds a patent on VWF-cleaving protease, and has received lecture fees or congress travel support from Siemens, Roche, Albynx-Sanofi, Bayer, and Alexion. F.P. and K.V. are members of the Scientific Advisory Boards for Ablynx-Sanofi and Shire-Takeda. P.C. is a member of the Advisory Boards for Ablynx-Sanofi, Alexion, Octapharma, and Cerus. The remaining authors declare no competing financial interests.
Correspondence: Karen Vanhoorelbeke, Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven Campus Kulak Kortrijk, Etienne Sabbelaan 53, B-8500 Kortrijk, Belgium; e-mail: karen.vanhoorelbeke@kuleuven.be.
REFERENCES
Author notes
E.R. and A.-S.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal