In this issue of Blood, Roose and colleagues present the role of open and closed forms of the metalloprotease ADAMTS13, expanding the understanding of the pathological role of antibodies related to immune-mediated thrombotic thrombocytopenic purpura (iTTP) while also providing an additional laboratory marker to monitor disease.1
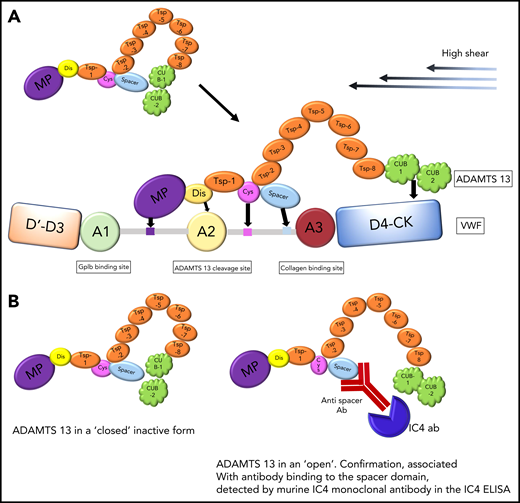
(A) Physiological action of ADAMTS13, which binds via CUB domains to D4-CK region of VWF under high shear stress. Further exosite binding of ADAMTS13 to VWF ensures alignment before VWF cleavage. (B) A cartoon to reflect the closed and open forms of ADAMTS13. ADAMTS13 is shown in a closed inactive form and an open confirmation, associated with antibody binding to the spacer domain, as detected by murine IC4 monoclonal antibody in the IC4 ELISA.
(A) Physiological action of ADAMTS13, which binds via CUB domains to D4-CK region of VWF under high shear stress. Further exosite binding of ADAMTS13 to VWF ensures alignment before VWF cleavage. (B) A cartoon to reflect the closed and open forms of ADAMTS13. ADAMTS13 is shown in a closed inactive form and an open confirmation, associated with antibody binding to the spacer domain, as detected by murine IC4 monoclonal antibody in the IC4 ELISA.
iTTP presents acutely and is confirmed by a severe deficiency of ADAMTS13 activity (<10 IU/dL) and the presence of immunoglobulin G (IgG) autoantibodies to ADAMTS13.2 The antibodies may have an inhibitory action, but those patients with the most severe disease also appear to have increased antibody mediated ADAMTS13 clearance.3 Therapy, principally ADAMTS13 enzyme replacement (through plasma exchange) and immunosuppression (typically steroids and anti-CD20 monoclonal therapy), is aimed at eliminating anti-ADAMTS13 antibodies and normalizing ADAMTS13 activity. iTTP is a chronic disease requiring monitoring of patients initially to prevent an acute relapse but, in the longer term, intervening with elective, prophylactic immunosuppressive therapy before ADAMTS13 activity levels are undetectable (<10IU/dL).4 Currently available assays measure ADAMTS13 activity and total IgG antibody. ADAMTS13 antigen levels are also useful, but availability is limited.
ADAMTS13 is a multidomain protein. Physiologically, ADAMTS13 is in a “closed” form. ADAMTS13 docks to the D4 region of von Willebrand factor (VWF), and under high shear, separation of the spacer-CUB regions is required for von Willebrand cleavage5 (see figure panel A). A number of groups have previously demonstrated that antibodies in thrombotic thrombocytopenic purpura (TTP) bind almost exclusively to the spacer region.6,7
Roose et al have previously reported a murine monoclonal antibody, IC4, which binds to a cryptic site in the spacer domain that is not accessible unless ADAMTS13 is in the open form. The IC4 enzyme-linked immunosorbent assay (ELISA) detects the presence of spacer antibodies, which cause ADAMTS13 to remain in an open structure8 (see figure panel B). Within the current publication, using iTTP samples with ADAMTS13 autoantibodies and healthy control samples, they confirmed their previous experiments; all but one of the iTTP samples were in the open ADAMTS13 configuration, and in healthy controls, as expected, ADAMTS13 was in a closed form. Of note, there is a minimum ADAMTS13 antigen level (0.03 μg/mL) that is required for the assay to be undertaken. Next, purified ADAMTS13 IgG from iTTP cases, added to pooled normal plasma containing closed ADAMTS13, resulted in an open ADAMTS13 confirmation in >80% of cases, substantiating the ability of antibodies to bind to the spacer region of ADAMTS13, detected in the IC4 ELISA.
A large cohort of iTTP patients, acute and in clinical remission, with varying ADAMTS13 activity levels were examined. In patients with activity levels <50%, the open ADAMTS13 confirmation was detected in >90% of samples. However, in patients with ADAMTS13 activity >50%, one-third still had detectable open form, suggesting residual antibody binding to the spacer region.
In patients with a decreasing ADAMTS13 activity monitored to identify the need for elective rituximab therapy, total ADAMTS13 antibody levels are not always measurable. However, using the IC4 ELISA, antibody may be detected, with an open ADAMTS13 confirmation. The authors suggest and present in a case followed over 5 years, that this assay can be used to detect subclinical disease. Why are antibodies to ADAMTS13 not detected when there is a significant reduction in ADAMTS13 activity yet patients respond to immunosuppressive therapy? Roose and colleagues postulate autoantibodies are bound to and cleared with ADAMTS13, so the total IgG antibody level may not increase, but antibody present within the spacer domain may be detected utilizing this new methodology.
Why is this work important in the understanding of the pathophysiology of TTP? Firstly, it provides confirmation that antibodies to ADAMTS13 alter its configuration and therefore its physiological function in von Willebrand cleavage. Secondly, these antibodies are not only present within the acute situation but also detectable as ADAMTS13 activity increases, suggesting inadequate antibody clearance. Finally, in patients whose ADAMTS13 activity starts to decrease from the normal range, spacer antibodies maybe detected by the IC4 ELISA before an increase in total ADAMTS13 IgG antibodies.
How does this aid clinical practice? Following an acute iTTP episode, confirmation of clearance of total ADAMTS13 IgG antibodies and a closed ADAMTS13 configuration by IC4 ELISA may avoid further unnecessary immunosuppression. Similarly, patients with detectable open ADAMTS13 despite an adequate increase in ADAMTS13 activity may require closer follow up and potentially the need for future elective therapy. Recognition of the open-closed confirmation of ADAMTS13 provides clinicians treating TTP patients with a further biomarker to monitor and identify subclinical disease.
Conflict-of-interest disclosure: M.S. has received speakers fees for and is a member of the advisory boards of Alexion, Takeda, Sanofi, and Novartis; and has received research funding from Shire.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal