In this issue of Blood, Dale and colleagues1 describe that WHIM patients, treated orally once daily with 400 mg of mavorixafor, a novel selective inhibitor of the CXCR4 receptor on bone marrow cells, had increased total white blood cells, neutrophils, and lymphocyte counts, as well as reduced infection rates and decreased wart numbers (see figure).
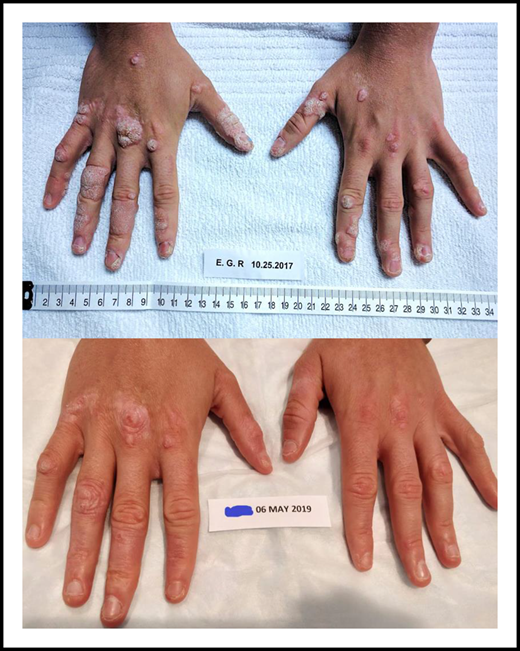
Reductions of warts on the hands of 1 participant in the mavorixafor trial. The upper panel shows the hands prior to start of mavorixafor treatment; the lower panel after completing 18 months of mavorixafor treatment. See Figure 5C-D in the article by Dale et al that begins on page 2994.
Reductions of warts on the hands of 1 participant in the mavorixafor trial. The upper panel shows the hands prior to start of mavorixafor treatment; the lower panel after completing 18 months of mavorixafor treatment. See Figure 5C-D in the article by Dale et al that begins on page 2994.
Sixteen years ago, a young woman came to me for evaluation of neutropenia and recurrent upper respiratory tract infections to see whether treatment with filgrastim might alleviate her symptoms.
She had already been started on subcutaneous gammaglobulins because of hypogammaglobulinemia and the infections. When holding out her hand to say hello, I noticed numerous warts on it. I said, “I believe you are suffering from WHIM syndrome.” How could I so unbashfully leap to this diagnosis? The reason was simple; I had read, by chance, a description of this syndrome and the causative mutation just a few days ago.2 Indeed, she harbored the mutation in the CXCR4 gene typical for WHIM, conferring this dominant autosomal inherited immunodeficiency syndrome. She barely tolerated filgrastim treatment because of bone pain and awaits an oral drug to combat her symptoms. Mavorixafor may be an answer, according to the article by Dale et al.
WHIM (OMIM 1836700) is caused by mutations leading to a gain-of-function in the CXCR4 receptor on bone marrow cells. This confers the typical findings for which WHIM is the acronym: warts, hypogammaglobulinemia, infections, and myelokathexis (although not all patients present with all symptoms, and a few phenocopies display mutations in the CXCR2 gene3 ). Although warts and genital condylomata are driven by undue susceptibility to papilloma virus, myelokathexis refers to the presence of numerous apoptotic neutrophils in the bone marrow that are not able to leave this compartment because the gate to the circulating blood is closed. The reason is the hyperactivity of the CXCR4/CXCL12 axis which, together with the CXCR2/CXCL2 axis, regulates emigration of neutrophils, as well as other cells, over endothelial barriers.3,4 Hypogammaglobulinemia is probably linked to reduced B-lymphocyte function/migration, also mirrored as lymphopenia in the blood. Other aspects of the disorder are monocytopenia, a propensity to develop malignancies, primarily lymphomas, as well as sequelae of frequent infections (eg, deafness, bronchiectasis).
For a long time, filgrastim and gammaglobulin substitution were the major treatments for WHIM, together with antibiotics.5 That treatment raised the blood neutrophil counts and gammaglobulin levels but had little effect on warts and lymphopenia. Some years ago, plerixafor (a specific CXCR4 inhibitor approved for hematologic stem cell mobilization), given twice daily subcutaneously, was introduced as a specific means to dampen the overactivity of the CXCR4 molecule.6 With plerixafor, neutropenias and lymphopenias were reduced, and regression of warts was observed. However, the need for twice-daily injections (due to short blood half-life) is a concern for compliance with plerixafor.
Dale and colleagues now report that the oral and specific CXCR4 inhibitor mavorixafor, given for up to ∼2 years, raised the absolute blood neutrophil, monocyte, and lymphocyte counts substantially, reduced the cutaneous number of warts by 75%, and decreased the annual infection rate from 4.6 to 2.3 episodes, with excellent tolerability. A phase 3 trial of mavorixafor is underway; it will provide more information about the possible differences in efficacy and safety compared with plerixafor.
There are other interesting aspects of CXCR4 inhibition in addition to the rare WHIM syndrome. Some solid tumors and lymphomas, particularly Waldenström macroglobulinemia, have recently been associated with somatic mutations in the CXCR4 gene, being WHIM-like. Although the relevance of these mutations for clinical presentation and survival, as well as their relationship with resistance to chemotherapy (eg, ibrutinib), are still unresolved issues, new treatment alternatives open up for these diseases.7 Trials are underway using plerixafor or mavorixafor for treatment of lymphomas with the WHIM-like mutation (eg, NCT04274738).
Despite these advances in the treatment of WHIM syndrome, a number of questions remain. Will there be safety issues related to long-term, and even lifelong, treatment with mavorixafor that cannot be foreseen now? Will immunoglobulin production normalize? How can the impressive (and possibly underestimated) 75% reduction in warts be increased to 100%? Is it a matter of extending treatment time, or is something additional needed? Is there also a reduction in genital condylomata? On the same note, is the burden of papilloma virus reduced by mavorixafor treatment? Another question is whether the malignancies associated with the WHIM syndrome will be prevented or, at least, reduced in frequency or severity. Because WHIM is such a rare disorder, combined efforts from all involved physicians are needed to increase awareness of this syndrome to offer novel treatments for these patients. Time will tell.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal