Key Points
Risk stratification and early intensification with high-dose cytarabine improves EFS for infants with KMT2A-r ALL.
Early MRD clearance is a strong predictor of favorable outcome in infants with ALL.
Abstract
The prognosis for infants with acute lymphoblastic leukemia (ALL), particularly those with KMT2A gene rearrangement (KMT2A-r), is dismal. Continuous efforts have been made in Japan to investigate the role of hematopoietic stem cell transplantation (HSCT) for infants with KMT2A-r ALL, but improvement in outcome was modest. In the Japanese Pediatric Leukemia/Lymphoma Study Group MLL-10 trial, infants with ALL were stratified into 3 risk groups (low risk [LR], intermediate risk [IR], and high risk [HR]) according to KMT2A status, age, and presence of central nervous system leukemia. Children’s Oncology Group AALL0631 modified chemotherapy with the addition of high-dose cytarabine in early intensification was introduced to KMT2A-r patients, and the option of HSCT was restricted to HR patients only. The role of minimal residual disease (MRD) was also evaluated. Ninety eligible infants were stratified into LR (n = 15), IR (n = 19), or HR (n = 56) risk groups. The 3-year event-free survival (EFS) rate for patients with KMT2A-r ALL (IR + HR) was 66.2% (standard error [SE], 5.6%), and for those with germline KMT2A (KMT2A-g) ALL (LR), the 3-year EFS rate was 93.3% (SE, 6.4%). The 3-year EFS rate was 94.4% (SE, 5.4%) for IR patients and 56.6% (SE, 6.8%) for HR patients. In multivariable analysis, female sex and MRD ≥0.01% at the end of early consolidation were significant factors for poor prognosis. Risk stratification and introduction of intensive chemotherapy in this study were effective and were able to eliminate HSCT for a subset of infants with KMT2A-r ALL. Early clearance of MRD seems to have translated into favorable outcomes and should be incorporated into risk stratifications in future trials. This trial was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) as #UMIN000004801.
Medscape Continuing Medical Education online
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1894.
Disclosures
Associate Editor Selina M. Luger received grants for clinical research from ARIAD Pharmaceuticals, Inc., BioSight Ltd., F. Hoffmann-La Roche Ltd, KURA Biotech, and Onconova Therapeutics, Inc. and honoraria from Acceleron Pharma, Agios Pharmaceuticals, Bristol Myers Squibb Company, Daiichi Sankyo, Inc., and Pfizer Inc. CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, and the authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the outcomes of infants with a higher-risk KMT2A gene rearrangement (KMT2A-r) acute lymphoblastic leukemia (ALL) and lower-risk germline KMT2A (KMT2A-g) ALL who were enrolled in the Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG) trial MLL-10
Determine the impact of minimal residual disease and other prognostic factors on the outcomes of infants with KMT2A-r ALL and KMT2A-g ALL who were enrolled in the JPLSG trial MLL-10
Identify clinical implications of the outcomes of infants with KMT2A-r ALL enrolled in the JPLSG trial MLL-10
Release date: October 15, 2020; Expiration date: October 15, 2021
Introduction
Acute lymphoblastic leukemia (ALL) in infants is biologically and clinically segregated into 2 subtypes: 1 with rearrangement of the KMT2A (MLL) gene that accounts for 80% of the cases and 1 with germline KMT2A gene.1 Outcomes in infants with KMT2A-rearranged (KMT2A-r) ALL are particularly poor, with reported outcomes of less than 50% event-free survival (EFS) rates in the major study groups worldwide.2,3 Attempts to improve outcomes by performing allogeneic hematopoietic stem cell transplantation (HSCT) for all infants with KMT2A-r ALL have been carried out in Japan since the late 1990s, but the improvement was modest.4-6 In addition, there is growing evidence that HSCT is beneficial only for the subset of infants with KMT2A-r ALL with additional high-risk (HR) features: younger age (<6 months old) with a high white blood cell count (WBC) (≥300 000/μL) or those who are poor responders to the initial prednisolone prephase.7,8 Even if HSCT is successful, risk of late effects continues to be an issue for a substantial number of survivors.9
With this background, the Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG) planned a nationwide clinical trial to improve the outcomes of infants with KMT2A-r ALL by introducing intensive chemotherapy and at the same time limiting the indication for HSCT to only those with HR features. Along with the main study, the role of minimal residual disease (MRD) was prospectively evaluated.
Methods
Patients
Patients for the JPLSG MLL-10 trial were recruited between January 2011 and December 2015. Eligible criteria included diagnosis of ALL (B-cell/myeloid mixed phenotype acute leukemia [MPAL] was eligible but not T-cell ALL, T-cell/myeloid MPAL, mature B-cell ALL, or Philadelphia chromosome–positive ALL) and age younger than 1 year at diagnosis. Written informed consent was obtained from the patients’ guardians according to the Declaration of Helsinki, and institutional review board approval was obtained for all aspects of the study.
Risk group stratification, treatment, and monitoring
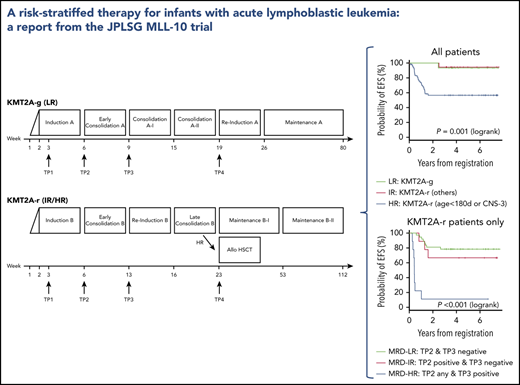
On the basis of combined analyses of studies MLL96 and MLL98,9 patients were stratified into 1 of 3 risk groups: low risk (LR), germline KMT2A (KMT2A-g); intermediate risk (IR), other KMT2A-r; and HR, KMT2A-r and age younger than 180 days at diagnosis or presence of central nervous system (CNS) leukemia (defined as CNS-3: ≥5 WBCs per μL in cerebral spinal fluid with leukemic blasts) evaluated on day 8 after the 7-day prednisolone prephase. The KMT2A gene configuration of each patient was determined with fluorescence in situ hybridization of a KMT2A gene split signal.
The details of the therapeutic regimen used in the MLL-10 study are described in supplemental Tables 2, 3, and 4 and supplemental Figure 1, available on the Blood Web site. The prednisolone response was evaluated on day 8, and a prednisolone good responder (PGR) was defined as an infant with a peripheral blood blast count <1000/μL; a prednisolone poor responder (PPR) was defined as an infant with a peripheral blood blast count of ≥1000/μL. The chemotherapy regimen used for the LR group (regimen A; supplemental Table 2) was based on the MLL96/MLL98 chemotherapy for KMT2A-g infant ALL.10 In contrast, the chemotherapy regimen for the IR and HR groups (regimen B; supplemental Table 3) was based on the Children’s Oncology Group (COG) AALL0631 chemotherapy with the following modifications11 : addition of high-dose cytarabine and Escherichia coli L-asparaginase in the early consolidation phase and use of native asparaginase because of the unavailability of the pegylated formula in Japan. AALL0631 backbone chemotherapy was selected for 2 reasons: (1) JPLSG did not have experience with treating KMT2A-r ALL infants with chemotherapy only, so it seemed more feasible to import an established protocol, and (2) although the plan of conducting a joint study between COG and JPLSG failed because of a new plan for the COG to test the FLT3 inhibitor lestaurtinib, it seemed reasonable to open a different trial, but with the same chemotherapy backbone so the results would be comparable. Complete remission (CR) was morphologically defined as <5% residual leukemic blasts in the bone marrow with regeneration of hematopoiesis and no evidence of leukemia elsewhere, which was evaluated at the end of the early consolidation course regardless of which regimen was used. Indication for HSCT was restricted to the HR group. The protocol-specific conditioning was a non-irradiation myeloablative regimen with busulfan (dose determined by an individual pharmacokinetic test measured centrally at Shinshu University Hospital), etoposide, and cyclophosphamide as previously described (supplemental Table 4).6,12
These aggressive supportive care guidelines were a part of the study protocol: full hospitalization throughout the treatment (except for maintenance phases), exchange transfusion guidelines for hyperleukocytosis, introduction of rasburicase for tumor lysis syndrome, intensive prophylactic use of granulocyte colony-stimulating factor, intensive empirical antimicrobial therapy, aggressive use of antifungal prophylaxis, intensive prophylactic intravenous immunoglobulin supplementation, and prophylactic use of palivizumab to prevent respiratory syncytial virus infection after the additional label in Japan for immunodeficiency in 2013.13
Central monitoring was performed for each patient twice per year using an electronic data capturing system during the study period. Expedited reporting was mandatory for all grade 4 and unexpected grade 3 nonhematologic adverse events (evaluated by Common Terminology Criteria for Adverse Events, version 3), all grade 5 events, and events that resulted in persistent and/or significant disability. Routine reporting was required for all grade 3 or greater adverse events.
MRD study
MRD in bone marrow specimens was prospectively evaluated at certain time points (supplemental Figure 1) using 3 different methods in each of the central reference laboratories. Flow cytometric MRD (FCM-MRD) was measured at Mie University using 4-color flow cytometry with marker combinations that allowed detection of leukemia with a sensitivity of ≤0.01%. The detailed method of FCM-MRD analysis is described in supplemental Table 5. Instances of FCM-MRD ≥0.01% and indication of leukemic cell clustering were regarded as positive. MRD targeting leukemia-specific genomic rearrangements of immunoglobulin (Ig) and/or T-cell receptor (TCR) using reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) (Ig/TCR PCR-MRD) was measured at Aichi Medical University, which is a certified laboratory of the European Study Group on MRD detection in ALL (EuroMRD).14-16 MRD was measured in each patient if it could be measured with a sensitivity of at least 10−4 and a quantitative range of at least 10−3. Ig/TCR PCR-MRD positivity was defined as ≥5 × 10−4. PCR-MRD targeting KMT2A chimeric fusion transcripts (KMT2A-fusion PCR-MRD) including KMT2A-AFF1, KMT2A-AFDN, KMT2A-MLLT3, and KMT2A-MLLT1 was measured at an SRL Inc. laboratory. KMT2A-fusion PCR-MRD positivity was defined as ≥50 copies per μg RNA.
Statistical analyses
Data from MLL-10 were current as of March 30, 2019, with a median follow-up of 5.4 years (range, 1.5-7.8 years) for living patients. The primary end point of the study was 3-year EFS rate for patients with KMT2A-r ALL. EFS was defined as the time from trial registration to the first event (failure to achieve CR, relapse, secondary malignancy, including lineage switch to acute myeloid leukemia [AML], or death as a result of any cause). The study was regarded as effective if the lower limit of the computed 95% confidence interval in 3-year EFS rates of KMT2A-r patients exceeded 40%, the null hypothesis of the trial. The secondary end points regarding efficacy were CR rate, bone marrow status at the end of initial induction, and EFS and overall survival (OS) rates in the total study cohort and among subgroups. OS was defined as the length of time from the trial registration to death as a result of any cause. The secondary end points regarding toxicity were grade 3 or greater adverse events, feasibility of regimen B for IR or HR patients, and correlation between busulfan pharmacokinetics and HSCT-related adverse events for HR patients. Follow-ups of the patients who discontinued the trial therapy were continued and were included in the analyses, unless their consent to the trial participation was withdrawn.
The EFS and OS rates were estimated with the Kaplan-Meier method and standard errors (SEs) were determined with the Greenwood formula and were then compared with the log-rank test. Variables including sex, WBC, age at diagnosis, KMT2A-r subtypes, initial CNS status, prednisolone response, bone marrow evaluation at end of initial induction, and MRD were examined in the univariable analyses. Cox proportional hazards regression was used to identify the risk factors associated with the EFS rate, and the variables that were significant at P < .1 were included in the model. Variables significantly associated with EFS were then identified by a significance level of 0.05. All P values were 2-sided, and no statistical adjustment was made for multiple testing. All data analyses were performed with STATA Release 14 statistical software (StataCorp, College Station, TX).
Results
Patient characteristics and overall outcomes
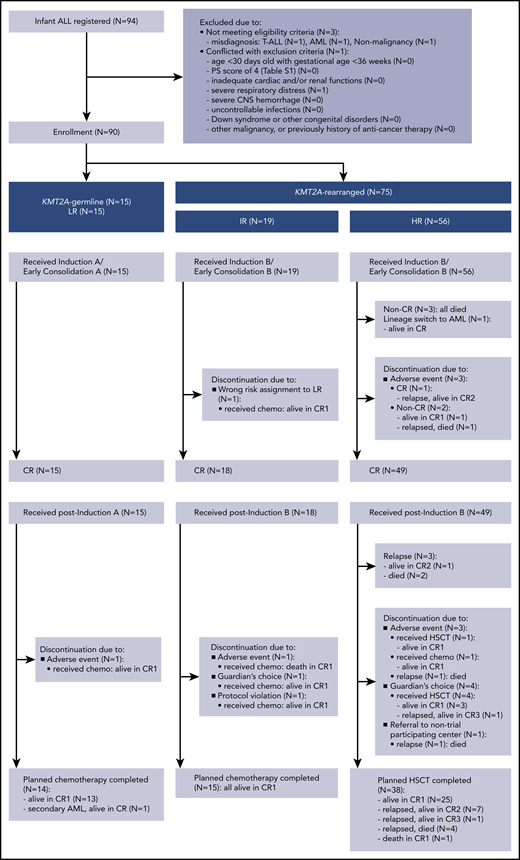
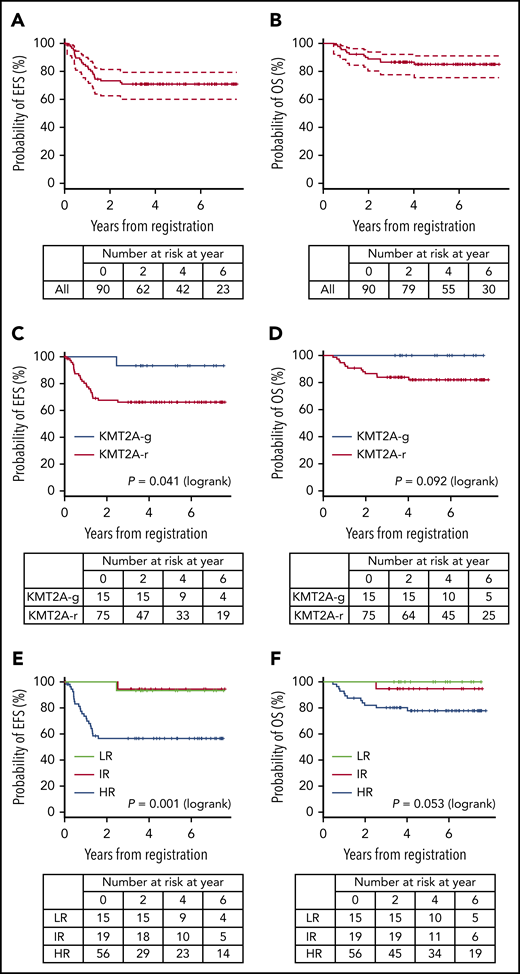
A total of 94 infants from 53 of 120 centers that participated in the trial were registered in the trial. Four patients were excluded from further analyses: 3 with the wrong diagnosis and 1 with severe respiratory distress before starting therapy. In all, 90 infants were eligible: 15 LR (17%), 19 IR (21%), and 56 HR (62%). Figure 1 presents the CONSORT flow diagram and Table 1 presents patient characteristics. Infants with KMT2A-r ALL had higher WBC, lower age distribution, more CD10– immunophenotypes, and higher rates of CNS leukemia compared with the KMT2A-g infants. Notably, the proportion of infants age younger than 180 days was high, as it was in the previous MLL03 study cohort. More than half the KMT2A-r patients had t(4;11)/KMT2A-AFF1 cytogenetics. The 3-year EFS and OS rates of all 90 patients were 70.9% (SE, 4.9%) and 86.6% (SE, 3.6%), respectively; the 5-year EFS and OS rates were 70.9% (SE, 4.9%) and 85.0% (SE, 3.9%), respectively (Figure 2A-B).
Characteristics of patients in MLL-10
| Characteristic . | All . | KMT2A-g . | KMT2A-r . | ||
|---|---|---|---|---|---|
| LR (n = 15) . | IR (n = 19) . | HR (n = 56) . | IR + HR (n = 75) . | ||
| No. (%) . | No. (%) . | No. . | No. . | No. (%) . | |
| Sex | |||||
| Male | 47 (52) | 10 (67) | 13 | 24 | 37 (49) |
| Female | 43 (48) | 5 (33) | 6 | 32 | 38 (51) |
| Age at diagnosis, days | |||||
| 0-89 | 22 (24) | 1 (7) | 0 | 21 | 21 (28) |
| 90-179 | 37 (41) | 2 (13) | 0 | 35 | 35 (47) |
| 180-365 | 31 (34) | 12 (80) | 19 | 0 | 19 (25) |
| WBC × 109/L at diagnosis | |||||
| <100 | 44 (49) | 12 (80) | 12 | 20 | 32 (43) |
| 100 to <300 | 19 (21) | 3 (20) | 2 | 14 | 16 (21) |
| ≥300 | 27 (30) | 0 (0) | 5 | 22 | 27 (36) |
| Immunophenotype | |||||
| B-lineage CD10– | 62 (69) | 1 (7) | 14 | 47 | 61 (81) |
| B-lineage CD10+ | 28 (31) | 14 (93) | 5 | 9 | 14 (19) |
| CNS involvement* | |||||
| Positive (CNS-3) | 10 (11) | 0 (0) | 0 | 10 | 10 (13) |
| Negative (CNS-1 or CNS-2) | 79 (88) | 15 (100) | 19 | 45 | 64 (85) |
| Unknown | 1 (1) | 0 (0) | 0 | 1 | 1 (1) |
| KMT2A status | |||||
| Germline | 15 (17) | 15 (100) | 0 | 0 | — |
| Rearranged | 75 (83) | — | 19 | 56 | 75 (100) |
| t(4;11)/KMT2A-AFF1 | — | 11 | 28 | 39 (52) | |
| t(9;11)/KMT2A-MLLT3 | — | 3 | 3 | 6 (8) | |
| t(11;19)/KMT2A-MLLT1 | — | 1 | 14 | 15 (20) | |
| Other KMT2A-r | — | 4 | 11 | 15 (20) | |
| Prednisolone response | |||||
| PGR | 60 (67) | 12 (80) | 17 | 31 | 48 (64) |
| PPR | 30 (33) | 3 (20) | 2 | 25 | 27 (36) |
| Characteristic . | All . | KMT2A-g . | KMT2A-r . | ||
|---|---|---|---|---|---|
| LR (n = 15) . | IR (n = 19) . | HR (n = 56) . | IR + HR (n = 75) . | ||
| No. (%) . | No. (%) . | No. . | No. . | No. (%) . | |
| Sex | |||||
| Male | 47 (52) | 10 (67) | 13 | 24 | 37 (49) |
| Female | 43 (48) | 5 (33) | 6 | 32 | 38 (51) |
| Age at diagnosis, days | |||||
| 0-89 | 22 (24) | 1 (7) | 0 | 21 | 21 (28) |
| 90-179 | 37 (41) | 2 (13) | 0 | 35 | 35 (47) |
| 180-365 | 31 (34) | 12 (80) | 19 | 0 | 19 (25) |
| WBC × 109/L at diagnosis | |||||
| <100 | 44 (49) | 12 (80) | 12 | 20 | 32 (43) |
| 100 to <300 | 19 (21) | 3 (20) | 2 | 14 | 16 (21) |
| ≥300 | 27 (30) | 0 (0) | 5 | 22 | 27 (36) |
| Immunophenotype | |||||
| B-lineage CD10– | 62 (69) | 1 (7) | 14 | 47 | 61 (81) |
| B-lineage CD10+ | 28 (31) | 14 (93) | 5 | 9 | 14 (19) |
| CNS involvement* | |||||
| Positive (CNS-3) | 10 (11) | 0 (0) | 0 | 10 | 10 (13) |
| Negative (CNS-1 or CNS-2) | 79 (88) | 15 (100) | 19 | 45 | 64 (85) |
| Unknown | 1 (1) | 0 (0) | 0 | 1 | 1 (1) |
| KMT2A status | |||||
| Germline | 15 (17) | 15 (100) | 0 | 0 | — |
| Rearranged | 75 (83) | — | 19 | 56 | 75 (100) |
| t(4;11)/KMT2A-AFF1 | — | 11 | 28 | 39 (52) | |
| t(9;11)/KMT2A-MLLT3 | — | 3 | 3 | 6 (8) | |
| t(11;19)/KMT2A-MLLT1 | — | 1 | 14 | 15 (20) | |
| Other KMT2A-r | — | 4 | 11 | 15 (20) | |
| Prednisolone response | |||||
| PGR | 60 (67) | 12 (80) | 17 | 31 | 48 (64) |
| PPR | 30 (33) | 3 (20) | 2 | 25 | 27 (36) |
PGR, prednisolone good responder; PPR, prednisolone poor responder.
CNS-3, ≥5 WBCs per μL in cerebral spinal fluid (CSF) with blasts; CNS-2, <5 WBCs per μL in CSF with blasts; CNS-1, absence of blasts in CSF regardless of the number of WBCs.
EFS and OS. (A-B) EFS and OS of all patients, (C-D) EFS and OS according to KMT2A status, and (E-F) EFS and OS according to the MLL-10 risk groups.
EFS and OS. (A-B) EFS and OS of all patients, (C-D) EFS and OS according to KMT2A status, and (E-F) EFS and OS according to the MLL-10 risk groups.
Outcomes of infants with KMT2A-r ALL
The 3-year EFS and OS rates of the 75 patients with KMT2A-r ALL were 66.2% (SE, 5.6%) and 83.9% (SE, 4.3%), respectively; the 5-year EFS and OS rates were 66.2% (SE, 5.6%) and 82.0% (SE, 4.6%), respectively (Figure 2C-D). The 3-year and 5-year EFS and OS rates of the IR patients were 94.4% (SE, 5.4%) and 94.7% (SE, 5.1%), respectively. The 3-year EFS and OS rates of the HR patients were 56.6% (SE, 6.8%) and 80.2% (SE, 5.4%), respectively; the 5-year EFS and OS rates were 56.6% (SE, 6.8%) and 77.9% (SE, 5.7%), respectively (Figure 2E-F). In addition, outcomes of the patients according to the risk groups adopted in the Interfant-06 study are described in Table 2 and supplemental Figure 2.3
Comparison and outcomes of the patients categorized into the 3 risk groups in MLL-10 and Interfant-06
| MLL-10 risk group and definition . | Interfant-06 risk group and definition . | 3- and 5-year EFS rates, % (SE) . | ||
|---|---|---|---|---|
| LR KMT2A-g (n = 15) . | MR KMT2A-r without HR features (n = 42) . | HR KMT2A-r, age <6 m, and WBC ≧300 000/μL or PPR (n = 33) . | ||
| LR, KMT2A-g (n = 15) | 15 | — | — | 93.3 (6.4) |
| IR, KMT2A-r and age ≧180 d and no CNS leukemia (n = 19) | — | 19 | 0 | 94.4 (5.4) |
| HR, KMT2A-r and either age <180 d or CNS leukemia (n = 56) | — | 23* | 33 | 56.6 (6.8) |
| 3- and 5-year EFS rates, % (SE) | 93.3 (6.4) | 82.4 (6.0) | 45.2 (8.9) | |
| MLL-10 risk group and definition . | Interfant-06 risk group and definition . | 3- and 5-year EFS rates, % (SE) . | ||
|---|---|---|---|---|
| LR KMT2A-g (n = 15) . | MR KMT2A-r without HR features (n = 42) . | HR KMT2A-r, age <6 m, and WBC ≧300 000/μL or PPR (n = 33) . | ||
| LR, KMT2A-g (n = 15) | 15 | — | — | 93.3 (6.4) |
| IR, KMT2A-r and age ≧180 d and no CNS leukemia (n = 19) | — | 19 | 0 | 94.4 (5.4) |
| HR, KMT2A-r and either age <180 d or CNS leukemia (n = 56) | — | 23* | 33 | 56.6 (6.8) |
| 3- and 5-year EFS rates, % (SE) | 93.3 (6.4) | 82.4 (6.0) | 45.2 (8.9) | |
3-y and 5-y EFS rates of the patients categorized as MLL-10 HR but Interfant-06 MR was 72.7% (SE, 9.5%).
Among the 19 IR patients, 1 discontinued the protocol therapy before CR evaluation because of being assigned to the wrong risk group. Fifteen completed the chemotherapy, and 3 discontinued postinduction protocol therapy because of an adverse event (n = 1), guardian’s choice (n = 1), or protocol violation (n = 1). Notably, the IR patient who discontinued because of an asparaginase-induced allergic event developed severe cytomegalovirus infection as a result of secondary T-cell immunodeficiency after chemotherapy completion, similar to the reported case series by Geerlinks et al.17 This patient eventually died of infection, although immune reconstitution by HSCT was attempted. In summary, treatment failure observed among the 19 IR patients was 1 death in CR.
Three of the 56 HR patients did not achieve CR, and 1 patient experienced lineage switch to AML. Three patients discontinued the protocol therapy before CR evaluation because of adverse events. Among the 49 patients achieving CR, 38 received planned HSCT (32 from an unrelated cord blood donor, 5 from a related bone marrow donor, and 1 from a related peripheral blood stem cell donor), of whom 25 are in CR, 12 have relapsed, and 1 died in CR (died of transplant-related pulmonary disease after second HSCT because of graft failure). Three patients relapsed before the scheduled HSCT. Eight patients discontinued the protocol therapy, of whom 5 patients underwent HSCT in first CR (CR1): 5 are alive in CR and 3 have relapsed. Reasons for discontinuation were adverse events (n = 3), guardian’s choice (n = 4), and referral to a center that was not participating in the trial (n = 1). In total, 43 patients (87% of the 49 HR patients achieving CR) received HSCT in CR1, and 29 patients (67%) are alive in CR. In summary, treatment failures observed among the 56 HR patients were induction failure (n = 3), lineage switch to AML (n = 1), relapse (n = 18; extramedullary relapse, n = 2; bone marrow relapse, n = 10; combined relapse, n = 6), and death in CR (n = 1).
Grade 3 or greater adverse events observed in each treatment course for IR and HR patients are listed in supplemental Tables 7-1, 7-2, and 8. Hematologic toxicities and nonhematological toxicities, such as aspartate aminotransferase/alanine aminotransferase (AST/ALT) elevation and infectious adverse events such as febrile neutropenia, were common; however, chemotherapy for IR and HR patients seemed feasible. There were no early deaths and only 2 deaths in CR.
Outcomes of infants with KMT2A-r ALL by MRD
FCM-MRD in patients with KMT2A-r ALL was evaluable in 68 (97%) of 70 patients at time point 1 (TP1), 67 (97%) of 69 at TP2, and 58 (95%) of 61 at TP3 (supplemental Figure 1). The 3-year and 5-year EFS rates of the patients with positive MRD were significantly worse compared with those of MRD-negative patients at almost all time points (supplemental Figure 3). Ig/TCR PCR-MRD and KMT2A-fusion PCR-MRD were also prognostic (supplemental Figures 4 and 5), but the former could be evaluable only in 37 (53%) of 70 patients, and the latter could be measured in selected patients with specific KMT2A fusions; therefore, further analyses were performed using FCM-MRD results.
Among the 57 patients with KMT2A-r ALL and available FCM-MRD results at both TP2 and TP3, MRD risk groups were defined as follows: MRD-LR was defined as patients with negative MRD at both TP2 and TP3, MRD-IR was defined as patients with positive MRD at TP2 and negative MRD at TP3, and MRD-HR was defined as patients with positive MRD at TP3. The 3-year and 5-year EFS rates of each of the MRD risk groups are shown in Table 3 and supplemental Figure 6. All but 1 patient among MLL-10 IR patients were categorized as MRD-LR, but MLL-10 HR patients fell into 3 different MRD risk groups. Therapy failed for all 8 patients with both MLL-10 HR and MRD-HR: 3 did not achieve CR, 4 relapsed before the scheduled HSCT, and 1 relapsed after HSCT in CR1. Among the 33 MLL-10 HR patients who were actually MRD-LR or MRD-IR, 9 relapsed (pre-HSCT, n = 1; post-HSCT, n = 8), but the other 23 patients are alive in CR (there was 1 death in CR).
Flow cytometric MRD risk groups in infants with KMT2A-r ALL in the MLL-10 trial
| . | MLL-10 . | ||||
|---|---|---|---|---|---|
| Risk group . | IR no.* . | HR no.* . | Overall no.* . | 3-y and 5-y EFS rates, % (SE) . | P . |
| MRD-LR (negative at TP2 and TP3) | 15 (0) | 24 (6) | 39 (6) | 78.4 (6.8) | |
| MRD-IR (positive at TP2, negative at TP3) | 0 | 9 (3) | 9 (3) | 66.7 (15.7) | |
| MRD-HR (positive or negative at TP2, positive at TP3) | 1 (0) | 8 (8) | 9 (8) | 11.1 (10.5) | <.001 |
| Overall | 16 (0) | 41 (17) | 57 (17) | ||
| . | MLL-10 . | ||||
|---|---|---|---|---|---|
| Risk group . | IR no.* . | HR no.* . | Overall no.* . | 3-y and 5-y EFS rates, % (SE) . | P . |
| MRD-LR (negative at TP2 and TP3) | 15 (0) | 24 (6) | 39 (6) | 78.4 (6.8) | |
| MRD-IR (positive at TP2, negative at TP3) | 0 | 9 (3) | 9 (3) | 66.7 (15.7) | |
| MRD-HR (positive or negative at TP2, positive at TP3) | 1 (0) | 8 (8) | 9 (8) | 11.1 (10.5) | <.001 |
| Overall | 16 (0) | 41 (17) | 57 (17) | ||
TP2, time point 2 (at the end of induction B); TP3, time point 3 (at the end of early consolidation B).
Number in parentheses indicates the number of induction therapy failures or relapses.
Outcomes of infants with KMT2A-r ALL by different prognostic variables
The 3-year and 5-year EFS rates according to the different prognostic variables are presented in Table 4; female sex and younger age at diagnosis were significant, and PPR was marginally associated with a poor prognosis in the univariable analysis. In the multivariable analysis, female sex and young age at diagnosis were again associated with a poorer EFS rate (Table 5). However, the most powerful predictor of all was the end of early consolidation MRD with a hazard ratio of 82.961 (P < .001).
Univariable analysis of prognostic factors (other than MRD) in infants with KMT2A-r ALL
| Variable . | No. of patients . | 3-y and 5-y EFS rates, % (SE) . | P . |
|---|---|---|---|
| Sex | .046 | ||
| Male | 37 | 78.8 (7.1) | |
| Female | 38 | 55.3 (8.1) | |
| WBC × 109/L at diagnosis | .106 | ||
| <100 | 32 | 79.9 (7.4) | |
| 100 to < 300 | 16 | 60.0 (12.7) | |
| ≥300 | 27 | 53.8 (9.8) | |
| Age at diagnosis, days | .017 | ||
| 0-89 | 21 | 61.5 (11.5) | |
| 90-179 | 35 | 54.1 (8.5) | |
| 180-365 | 19 | 94.4 (5.4) | |
| KMT2A fusion | .416 | ||
| t(4;11)/KMT2A-AFF1 | 39 | 64.0 (7.7) | |
| t(9;11)/KMT2A-MLLT3 | 6 | 100 | |
| t(11;19)/KMT2A-MLLT1 | 15 | 60.0 (12.7) | |
| Other KMT2A-r | 15 | 63.6 (14.5) | |
| CNS status | .728 | ||
| CNS-1 | 42 | 69.0 (7.2) | |
| CNS-2 | 22 | 60.0 (11.0) | |
| CNS-3 | 10 | 63.5 (16.9) | |
| Unknown | 1 | — | |
| Prednisolone response | .071 | ||
| PGR | 48 | 73.9 (6.5) | |
| PPR | 27 | 52.1 (10.0) | |
| Bone marrow evaluation at the end of initial induction | .464 | ||
| M1 marrow | 64 | 66.6 (6.0) | |
| M2/M3 marrow | 5 | 53.3 (2.5) | |
| Unknown | 6 | — |
| Variable . | No. of patients . | 3-y and 5-y EFS rates, % (SE) . | P . |
|---|---|---|---|
| Sex | .046 | ||
| Male | 37 | 78.8 (7.1) | |
| Female | 38 | 55.3 (8.1) | |
| WBC × 109/L at diagnosis | .106 | ||
| <100 | 32 | 79.9 (7.4) | |
| 100 to < 300 | 16 | 60.0 (12.7) | |
| ≥300 | 27 | 53.8 (9.8) | |
| Age at diagnosis, days | .017 | ||
| 0-89 | 21 | 61.5 (11.5) | |
| 90-179 | 35 | 54.1 (8.5) | |
| 180-365 | 19 | 94.4 (5.4) | |
| KMT2A fusion | .416 | ||
| t(4;11)/KMT2A-AFF1 | 39 | 64.0 (7.7) | |
| t(9;11)/KMT2A-MLLT3 | 6 | 100 | |
| t(11;19)/KMT2A-MLLT1 | 15 | 60.0 (12.7) | |
| Other KMT2A-r | 15 | 63.6 (14.5) | |
| CNS status | .728 | ||
| CNS-1 | 42 | 69.0 (7.2) | |
| CNS-2 | 22 | 60.0 (11.0) | |
| CNS-3 | 10 | 63.5 (16.9) | |
| Unknown | 1 | — | |
| Prednisolone response | .071 | ||
| PGR | 48 | 73.9 (6.5) | |
| PPR | 27 | 52.1 (10.0) | |
| Bone marrow evaluation at the end of initial induction | .464 | ||
| M1 marrow | 64 | 66.6 (6.0) | |
| M2/M3 marrow | 5 | 53.3 (2.5) | |
| Unknown | 6 | — |
Multivariable analysis of prognostic factors for EFS in infants with KMT2A-r ALL
| Variable . | No. of patients . | Hazard ratio . | SE (%) . | 95% CI (%) . | P . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 27 | 1 | — | — | |
| Female | 31 | 7.070 | 5.691 | 1.460-34.241 | .015 |
| Age at diagnosis, days | |||||
| 0-89 | 14 | 1 | — | — | |
| 90-179 | 28 | 1.674 | 1.120 | 0.451-6.211 | .441 |
| 180-365 | 16 | 0.129 | 0.160 | 0.011-1.469 | .099 |
| Prednisolone response | |||||
| PGR | 39 | 1 | — | 58.7-84.3 | |
| PPR | 19 | 1.214 | 0.584 | 31.3-69.3 | .687 |
| FCM-MRD at TP3 | |||||
| Negative | 49 | 1 | — | 55.8-78.5 | |
| Positive | 9 | 82.961 | 73.356 | 5.2-75.3 | <.001 |
| Variable . | No. of patients . | Hazard ratio . | SE (%) . | 95% CI (%) . | P . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 27 | 1 | — | — | |
| Female | 31 | 7.070 | 5.691 | 1.460-34.241 | .015 |
| Age at diagnosis, days | |||||
| 0-89 | 14 | 1 | — | — | |
| 90-179 | 28 | 1.674 | 1.120 | 0.451-6.211 | .441 |
| 180-365 | 16 | 0.129 | 0.160 | 0.011-1.469 | .099 |
| Prednisolone response | |||||
| PGR | 39 | 1 | — | 58.7-84.3 | |
| PPR | 19 | 1.214 | 0.584 | 31.3-69.3 | .687 |
| FCM-MRD at TP3 | |||||
| Negative | 49 | 1 | — | 55.8-78.5 | |
| Positive | 9 | 82.961 | 73.356 | 5.2-75.3 | <.001 |
CI, confidence interval.
Outcomes of infants with KMT2A-g ALL
All 15 LR patients (KMT2A-g) achieved CR: 14 patients completed the planned chemotherapy of whom 13 are alive in CR, 1 developed therapy-related AML with KMT2A-MLLT3, and 1 discontinued protocol therapy because of an adverse event (Figure 1). Therefore, the 3-year and 5-year EFS and OS rates of the LR patients were 93.3% (SE, 6.4%) and 100%, respectively (Figure 2E-F); these results were consistent with the 5-year EFS and OS rates of 95.5% for the 22 infants with KMT2A-g ALL in the previous MLL96 and MLL98 studies.10
FCM-MRD was evaluated in 12 LR patients (no sample submission for 3 patients): MRD was negative in 11 patients at TP2 (no sample submission for 1 patient) and negative in 11 patients at TP3 (no sample submission for 1 different patient). In contrast to the KMT2A-r patients, Ig/TCR PCR-MRD could be evaluated in 13 (87%) of 15 patients; MRD was negative in all 13 patients at both TP2 and TP3.
Discussion
The outcomes of the patients in the MLL-10 trial demonstrated a significant improvement compared with the past Japanese infant ALL trials (supplemental Figure 7). In addition, the 3-year and 5-year EFS rates in each of the 3 Interfant-06 defined risk groups were higher than the 6-year EFS rates observed in the original Interfant-06 study, the largest and the most recent contemporary infant ALL study: 93.3% vs 73.9% for LR, 82.4% vs 44.5% for MR, and 45.2% vs 20.9% for HR.3 Although the follow-up period for MLL-10 is shorter, the comparison would still be reasonable, given that most events in the Interfant-06 cohort occurred within 3 years.
An additional limitation of the study is the smaller number of patients included in MLL-10 compared with the Interfant-06 study; therefore, caution is required when interpreting the results, and potential bias cannot be fully excluded. However, according to the registry data of the Japanese Society of Pediatric Hematology, which is estimated to represent 80% of all the hematologic cancer cases in Japan, 108 infant ALL cases were identified between 2006 and 2010.18 Considering this data, 90 cases per 5-year recruitment period in this study seems to cover a reasonable number of infants with ALL nationwide in terms of selection bias.
One of the characteristics of the MLL-10 study population is the high proportion of infants younger than age 6 months (65%), which is usually about 50% in the other collaborative studies, including Interfant-06.2,3 This high proportion of younger infants is consistent with that observed in the MLL03 study and may reflect potential unique biological characteristics of infant ALL in Japan.6 However, outcomes of younger infants are poorer and contradictory as an explanation of the favorable outcome. In addition, the outcomes of MLL-10 were markedly superior to those of the past Japanese studies, including MLL03, which were consistent with non-Japanese collaborative studies6,9 ; therefore, this potentially different biological profile is unlikely to explain the favorable outcomes observed in MLL-10. Patient characteristics other than age at diagnosis were comparable between the MLL-10 and the Interfant-06 cohorts.3
Both MLL-10 and Interfant-06 studies used the same induction chemotherapy. Differences in postinduction chemotherapy might explain the outcome difference, but that is also unlikely because the reported outcome of COG AALL0631 trial, the original trial that used the chemotherapy regimen used in MLL-10, was 37% for the 3-year EFS rate for KMT2A-r patients, which is comparable to the 36.4% EFS rate observed in Interfant-06.3,11 However, several modifications to the original COG chemotherapy are likely to have had an impact on the favorable outcomes in MLL-10 and thus contributed substantially to the improved overall outcome: more stringent criteria for dose reduction according to age, addition of high-dose cytarabine and L-asparaginase in early consolidation, and broader indications for HSCT.
Dose adjustments of chemotherapeutic drugs are usually used for infants according to their age; however, they are somewhat arbitrary because of the limited pharmacokinetic and pharmacodynamic data in infants. Moreover, these doses are generally based on tolerability rather than on therapeutic effect. Compared with MLL-10 cohorts, a wider range of patients in Interfant-06 received reduced chemotherapeutic dosing, such that infants younger than age 6 months and those age 6 to 12 months received two-thirds and three-quarters of the calculated doses in Interfant-06, respectively, whereas infants younger than age 2 months and those age 2 to 3 months received two-thirds and three-quarters of the calculated doses in MLL-10, respectively (supplemental Tables 2 and 3).3 Given the higher proportion of younger infants in the MLL-10 cohort compared with that in the Interfant-06 cohort, particularly among those with KMT2A-r, this dose reduction criterion led to intensified dosing for a substantial number of the infants in MLL-10, which might have had an impact on the favorable outcome.
The high sensitivity of infant ALL cells to cytarabine while being resistant to ALL key drugs such as corticosteroids and asparaginase is one of the key rationales for why infant ALL protocols include an AML-type treatment element such as cytarabine.19 Although introduction of AML chemotherapy blocks failed to demonstrate benefit in the Interfant-06 study, we believe that the addition of high-dose cytarabine and asparaginase is likely to have contributed to the favorable outcome in MLL-10 because this is one of the largest differences between MLL-10 chemotherapy and the original COG AALL0631 chemotherapy, and the AML blocks in the Interfant-06 study contain lower doses of cytarabine.3,11
Another difference between the MLL-10 cohort and the Interfant-06 cohort is the broader indication for HSCT in MLL-10 because of the difference in HR definition. In addition, most of the HR patients who achieved CR in MLL-10 (43 of 49) received HSCT, but only half (76 of 143) of the HR patients achieving CR in Interfant-06 received HSCT because of an early relapse: percentages of patients receiving HSCT in the total cohort were 52% (43 of 82) in MLL-10 vs 18% (111 of 605) in Interfant-06, and none in COG AALL0631.3,11 Recent evidence does not support the benefit of HSCT in infant ALL; however, the high percentage of continuous CR after HSCT (67%) in MLL-10 compared with the 4-year disease-free survival of 44.0% in Interfant-06 and the low percentage of HSCT-related death in MLL-10 (2%; 1 of 43) compared with 14.4% (16 of 111) in Interfant-06 are likely to have contributed to the favorable outcomes in MLL-10.
It is notable that among the 57 KMT2A-r patients in whom MRD was evaluable at both TP2 and TP3, 39 patients (68%) fell into MRD-LR and 48 patients (84%) had negative MRD at TP3. According to the data from the Interfant-99 study, the MRD level of <10−4 (measured by RT qPCR of rearranged Ig/TCR and KMT2A genes) was found in only about 30% of the KMT2A-r patients at TP2 (after the same induction as that in MLL-10) and about 60% of the patients at TP3 (after early consolidation with a combination of 6-mercaptopurine, high-dose methotrexate, intrathecals, and high-dose cytarabine plus L-asparaginase [MARMA]).20 Although the methodology of measuring MRD differs between the 2 studies, considerably higher MRD clearance may explain the high EFS and OS rates in the MLL-10 study.
We believe that aggressive supportive care played a certain role in this study. There were no early deaths in induction and early consolidation phases and only 2 deaths in CR in the postremission phases, and the grade 3 or greater toxicities observed were permissible. Compared with the previous MLL03 study, availability of rasburicase in Japan in 2009 was a major step because the rate of tumor lysis syndrome dropped from 40% to 20%, and no related deaths were observed in this study.6,21 Appropriate management of toxicities enabled us to treat the infants with high-intensive chemotherapy and should have contributed to the improved EFS and OS rates in MLL-10. However, this might not be the major factor for the favorable outcomes because the difference in the rate of death in CR between MLL-10 and Interfant-06 (2.2% vs 7.1%) is not large enough for its explanation.3
Finally, considering the above-mentioned comparisons between MLL-10 and Interfant-06, we speculate that the infants enrolled in the MLL-10 trial may have had a higher proportion of AML-like leukemia with higher sensitivity to chemotherapy, particularly to cytarabine. Thus, they benefitted from intensive chemotherapy derived from a narrower adaptation of age-dependent drug reduction criteria and the addition of a high-dose cytarabine-asparaginase combination in early consolidation, which resulted in early clearance of MRD. Together with satisfactory HSCT outcomes, the MLL-10 cohort might have ended up with remarkably high EFS and OS rates. Conversely, MRD clearance, particularly at the end of early consolidation, would be a powerful tool to predict outcome; therefore, MRD should be included in risk stratifications in future trials. However, one should be cautious about which MRD methodology is to be used, particularly for KMT2A-r patients. Considering the low availability of Ig/TCR PCR-MRD in infants with KMT2A-r ALL (explained by their immature Ig/TCR gene rearrangement patterns and the oligoclonality of leukemia), additional procedures such as use of FCM-MRD, combining PCR-MRD targeting KMT2A genomic breakpoints, or detecting Ig/TCR genomic breakpoints with a novel technique such as next-generation sequencing are necessary.20,22 In addition, early MRD clearance might be a useful surrogate marker for evaluating the efficacy of a given therapy for infants with KMT2A-r ALL.
Patients categorized in the conventional HR group and those with residual MRD are definitely the patients who could not be cured with conventional therapeutic approaches including HSCT, and therefore they should be treated by using a novel therapeutic approach. Whether MRD-selected LR and IR patients within the conventional HR category could successfully be treated without HSCT is an open question, but considering its potential late effects, the HSCT indication should be eliminated in the future. There are several classes of novel therapeutics that are expected to be active against infant KMT2A-r ALL, such as novel purine nucleoside analogs, epigenetic modifiers, BCL-2 inhibitors, menin inhibitors, and immunotherapies including blinatumomab and chimeric antigen receptor T-cell therapy.23-30 Nonetheless, these alternative approaches that incorporate novel therapeutics should be established in the near future.
The Japan Children’s Cancer Group (JCCG) is committed to sharing, with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by the JCCG steering committee based on scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the JPLSG and the Japan Children’s Cancer Group investigators involved in the MLL-10 trial; Yoshie Okano, Noriko Sato, and Kaori Nagai from the Organization for Supporting Clinical Research data center for managing trial data; and the COG investigators, particularly Stephen P. Hunger, Patrick Brown, and Bruce M. Camitta, for providing information on AALL0631 protocol. The authors also thank the medical editor from the Department of Education for Clinical Research of the National Center for Child Health and Development for his assistance with editing this manuscript.
This study was supported by a grant for clinical cancer research from the Ministry of Health, Labour, and Welfare of Japan (H23-KakushintekiGan-Ippan-014) (K.H.), grants for Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (15ck0106071h0002) (A.M.) and (18ck0106436h0001) (T.M.), a grant from the National Center for Child Health and Development (30-1) (D.T.), and a research grant from the Children’s Cancer Association of Japan.
Authorship
Contribution: D.T., T.M., A.O., Y.T., M.H., and K.K. conceived and designed the study; D.T., T.M., and T.I. reviewed the data analysis and interpretation and served as the main authors of the manuscript; A.M.S. and T.W. conducted the statistical analysis; T.D. performed immunodiagnostic and FCM-MRD analyses; T.H. and M.S. performed Ig/TCR PCR-MRD analysis; S.O. and M.H. performed the busulfan pharmacokinetic study; T.T. coordinated the molecular biology analyses; Y.A., A.I., and Y.K. recruited patients; D.T., T.M., A.M., K.H., and E.I. contributed to the financial and administrative support of the study; and all authors contributed to the conduct of the trial, reviewed the results, and provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daisuke Tomizawa, Division of Leukemia and Lymphoma, Children’s Cancer Center, National Center for Child Health and Development, 2-10-1 Okura, Setagaya-ku, Tokyo 157-8535, Japan; e-mail: tomizawa-d@ncchd.go.jp.