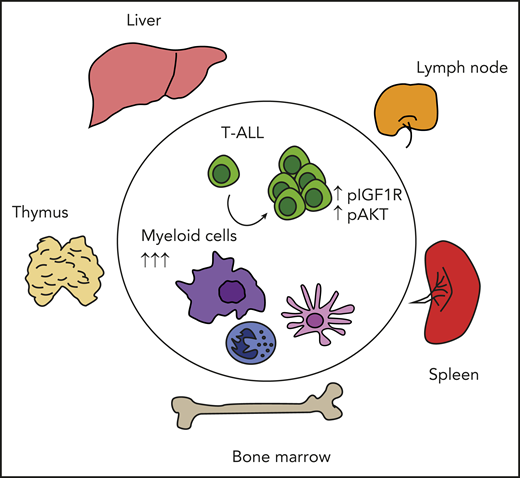
In this issue of Blood, Lyu et al tackle the fascinating question of identifying novel mediators of leukemia progression hidden in the intricate host environment.1 Particularly, they provide robust evidence for an essential role of myeloid cells in supporting T-cell acute lymphoblastic leukemia (T-ALL).
T-ALL cells depend on multiple myeloid cells to sustain prosurvival signals and expand in different tissues and organs. Myeloid cells are expanded in vivo upon T-ALL dissemination, and their in vivo depletion interferes with T-ALL progression and activation of prosurvival signals such as IGF1R and AKT.
T-ALL cells depend on multiple myeloid cells to sustain prosurvival signals and expand in different tissues and organs. Myeloid cells are expanded in vivo upon T-ALL dissemination, and their in vivo depletion interferes with T-ALL progression and activation of prosurvival signals such as IGF1R and AKT.
Acute leukemias are aggressive cancers occurring during both childhood and adulthood, with a poor overall prognosis. T-ALL arises from genetic alterations in T-cell precursors, causing developmental arrest and accumulation of T-cell blasts in the thymus, blood, bone marrow, and peripheral tissues.2 The role of extrinsic cues in supporting healthy and malignant hematopoiesis has recently received increasing attention.3,4 The definition of the T-ALL microenvironment is particularly difficult because of the highly motile nature of leukemic T cells and the involvement of multiple sites in the disease, including the thymus, bone marrow, spleen, and lymph nodes, as well as the liver and the central nervous system.
Ehrlich and colleagues have previously characterized the diffuse alteration of the thymic microenvironment in primary mouse models of T-ALL and found that tumor-associated dendritic cells were essential in supporting leukemic growth in vitro.5 In their new study,1 a transplantable model of the same disease enables the authors to characterize the microenvironment in peripheral organs, such as spleen, bone marrow, and liver. The results confirmed an overall deregulation of distinct myeloid subsets in the leukemic tissues.
Surprisingly, dendritic cells were dispensable for leukemia progression in vivo.1 This discrepancy with the previous report could be due to several reasons. First of all, the differences between in vitro and in vivo experimental systems could have an impact. Second, the 2 studies focus on different disease stages, with transplantable secondary leukemias being more aggressive, with a shorter latency and a potentially lower degree of dependency on supportive niches. In line with this hypothesis, the authors observed that the overall suppression of myeloid subsets was needed to cause a significant slowing of T-ALL progression.
Dendritic cells and macrophages are known as important components of the tumor immune microenvironment and can have a role in mediating immune suppression in both solid and hematological cancers.6 However, the supportive effect of myeloid cells on T-ALL is independent of the presence of adaptive immune response, while it seems to lean at least in part on boosting prosurvival signals such as IGF1R, an essential growth factor receptor for leukemia initiating cell activity,7 in the leukemic compartment. Nevertheless, it remains unclear whether the myeloid compartment is a direct source of ligands for such signaling.
The direct support function exerted by myeloid cells on T-ALL is not specific for 1 myeloid subset or 1 specific tissue, but it is a rather highly redundant feature. One could speculate that myeloid cells are likely to be a common feature of supportive microenvironment over different tissues and thus represents an essential component of the T-ALL niche (see figure). Further studies allowing deep molecular characterization and tissue-specific depletion of T-ALL–associated myeloid cells would be required to confirm this hypothesis.
The effect of T-ALL infiltration on myeloid cell phenotype and function remains to be determined. Recent work explored the remodeling of the bone marrow immune microenvironment at single-cell resolution in human acute myeloid leukemia8 and B-cell acute lymphoblastic leukemia.9 In the latter, a peculiar accumulation of nonclassical CSF1Rhigh/CD16+ monocytes showing an aberrant transcriptomic signature potentially linked with vascular damage was found.9 Both clinical studies have reported an inferior overall survival associated with an increase in specific markers and subtypes of myeloid cells. Along the same line, Lyu et al analyzed data from a large cohort of T-ALL patient-derived samples and observed a worse outcome associated with high monocytes and macrophage signatures.
In light of the multiple challenges encountered in designing an efficient immunotherapy against T-ALL,10 the work from Lyu et al opens a new chapter of investigation in the T-ALL immune niche, paving the way for mechanistic and clinical studies to further decipher the intimate relationship between T-ALL and the myeloid microenvironment and exploit it for therapeutic benefit.
Conflict-of-interest disclosure: The author declares no competing financial interests.