TO THE EDITOR:
Patients with end-stage chronic liver disease (ie, cirrhosis) acquire multiple and complex alterations in their hemostatic system. Recent insights in the hemostatic changes in patients with cirrhosis have indicated a balanced, but unstable hemostatic system in these patients with a risk for both bleeding and thrombotic complications including venous thromboembolism and portal vein thrombosis.1,2 Prevention and treatment of thrombotic events are a challenge because of a frequently prolonged baseline international normalized ratio and substantially decreased levels of antithrombin, impeding correct dosing and monitoring of vitamin K antagonists and heparins, respectively.3,4 There is very limited clinical experience with the new-generation direct oral anticoagulants (DOACs) in patients with cirrhosis because these patients were excluded from all clinical trials with these new agents. However, DOACs have potential advantages over vitamin K antagonists and heparins, such as the oral route of administration, the lack of requirement of laboratory monitoring, their mechanism of action, and the wider therapeutic window,4 which has resulted in increasing interest from the hepatology community.
DOACs are contraindicated in patients with advanced liver disease accompanied with coagulopathy and clinically relevant bleeding risk. Importantly, because all the DOACs are cleared by the liver and kidney, drug accumulation with a potentially increased bleeding risk is a main concern, although published pharmacokinetic studies have looked at a single dose only.5 In addition, in vitro studies have shown altered anticoagulant potency of DOACs in plasma from patients with cirrhosis. Nevertheless, DOACs have been used in attempts to treat portal vein thrombosis, venous thrombosis, and atrial fibrillation. Although the available safety data are encouraging, most studies were underpowered for efficacy end points.5-8
Here, we studied in vivo anticoagulant effects of the anti-Xa targeting DOAC edoxaban (60 mg once daily, administered for 7 consecutive days). Sixteen adult patients with an established diagnosis of cirrhosis were enrolled from the outpatient hepatology clinic of the University Medical Center Groningen, The Netherlands. In addition, 16 healthy volunteers were included as a control group. Cirrhosis had to be confirmed via FibroScan suggestive for F4 fibrosis, histology compatible with cirrhosis, or via diagnostic imaging. Characteristics of patients and controls are shown in Table 1. Exclusion criteria were the presence of malignancy, renal failure requiring intervention with drugs or dialysis (creatinine clearance of <50 mL/min); weight <60 kg; active infection; a known hereditary bleeding disorder; use of anticoagulant drugs in the past 10 days; use of cyclosporine, dronedarone, erythromycin, or ketoconazole; history of thrombotic disease; recent variceal bleeding or known varices grade 2 or 3; pregnancy; or HIV positivity. Additional exclusion criteria for the control group included the presence of known liver disease and a history of clinically relevant bleeding complications. The study protocol was approved by the local medical ethical committee (METc 2016/226), and was registered at Netherlands Trial Register (NTR6397). Written informed consent was obtained from each subject before inclusion in the study.
Baseline clinical and laboratory characteristics
| . | Patients (n = 16) . | Controls (n = 16) . |
|---|---|---|
| Age | 60 [48-65] | 49 [42-58]* |
| Female | 6 (38) | 7 (44) |
| Etiology of liver disease | ||
| Cholestatic liver disease | 4 | |
| Nonalcoholic steatohepatitis | 2 | |
| Alcoholic steatohepatitis | 4 | |
| Autoimmune hepatitis | 2 | |
| Hepatitis C virus | 1 | |
| Hemochromatosis | 1 | |
| Overlap syndromes | 2 | |
| BMI, kg/m2 | 28.7 [5] | 26.4 [4.4] |
| Diabetes | 4 (25) | 1 (6) |
| CVD | 5 (31) | 2 (13) |
| CTP A | 15 (94) | — |
| CTP B | 1 (6) | — |
| Ascites | 2 (13) | — |
| Encephalopathy | 0 | — |
| APTT, s | 34.4 [4.2] | 32.2 [2.7] |
| PT, s | 12.3 [2.4] | 11.0 [0.7]* |
| INR | 1.1 [0.2] | 1.0 [0.1]* |
| Fibrinogen, g/L | 2.8 [0.9] | 3.1 [0.7] |
| Prothrombin, % | 65 [15] | 102 [11]* |
| D-dimer, ng/mL | 517 [308-1049] | 185 [124-276]* |
| . | Patients (n = 16) . | Controls (n = 16) . |
|---|---|---|
| Age | 60 [48-65] | 49 [42-58]* |
| Female | 6 (38) | 7 (44) |
| Etiology of liver disease | ||
| Cholestatic liver disease | 4 | |
| Nonalcoholic steatohepatitis | 2 | |
| Alcoholic steatohepatitis | 4 | |
| Autoimmune hepatitis | 2 | |
| Hepatitis C virus | 1 | |
| Hemochromatosis | 1 | |
| Overlap syndromes | 2 | |
| BMI, kg/m2 | 28.7 [5] | 26.4 [4.4] |
| Diabetes | 4 (25) | 1 (6) |
| CVD | 5 (31) | 2 (13) |
| CTP A | 15 (94) | — |
| CTP B | 1 (6) | — |
| Ascites | 2 (13) | — |
| Encephalopathy | 0 | — |
| APTT, s | 34.4 [4.2] | 32.2 [2.7] |
| PT, s | 12.3 [2.4] | 11.0 [0.7]* |
| INR | 1.1 [0.2] | 1.0 [0.1]* |
| Fibrinogen, g/L | 2.8 [0.9] | 3.1 [0.7] |
| Prothrombin, % | 65 [15] | 102 [11]* |
| D-dimer, ng/mL | 517 [308-1049] | 185 [124-276]* |
Data are expressed as number (%), mean [SD], or median [IQR].
APTT, activated partial thromboplastin time; BMI, body mass index; CVD, cardiovascular disease; CTP, Child Turcotte Pugh; INR, international normalized ratio; PT, prothrombin time.
P < .05.
Blood samples were drawn by venipuncture twice at day 1 (baseline and 2 hours after the first dose) and once on day 3 and day 7 at peak level, 2 hours after ingestion of edoxaban. The blood was processed to platelet-poor plasma by double centrifugation at 2000g and 10 000g respectively for 10 minutes. Plasma was stored at −80°C until use.
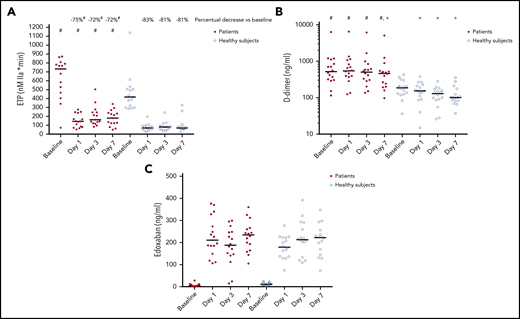
We estimated ex vivo plasma anticoagulant capacity by thrombomodulin-modified calibrated automated thrombinography.9 At baseline, the endogenous thrombin potential (ETP) was substantially higher in patients compared with controls (707 [482-789] nM IIa × min vs 403 [304-506] nM IIa × min, P < .05). On days 1, 3, and 7, ETP decreased in both patients and controls, but remained significantly and substantially higher in patients compared with the healthy subjects (Figure 1A). The percentual decrease of the ETP on edoxaban compared with baseline was significantly lower in patients compared with controls at all time points (P < .05). D-dimer levels decreased significantly over time in controls compared with baseline (P < .05), but remained similar compared with baseline in patients (Figure 1B). We estimated edoxaban plasma levels by edoxaban-calibrated anti-Xa assays on the ACL 300 TOP (Werfen, Breda, The Netherlands) with BIOPHEN heparin purchased from Nodia (Amsterdam, The Netherlands), calibrated with the BIOPHEN Edoxaban calibrator from Nodia. In patients and controls alike, anti-Xa levels remained relatively constant during the week of administration, with median plasma levels ∼200 ng/mL (Figure 1C). At day 3, 2 of the patients had particularly low edoxaban plasma levels, which may have been caused by lack of compliance. Two patients experienced a minor nosebleed. One patient decided not to participate any further after this event. Mild bruising mainly at the site of venipuncture was present in 4 patients and 5 healthy controls. No further adverse events occurred.
Anticoagulant effects of edoxaban in healthy volunteers and patients with cirrhosis. (A) Endogenous thrombin potential (ETP), (B) D-dimer levels, and (C) edoxaban-calibrated anti-Xa levels at baseline and in samples taken at 3 time points during administration of 60 mg edoxaban once daily to 16 patients with cirrhosis and 16 healthy controls. Horizontal lines represent medians. Values in patients and controls at a single time point were compared using Student t test or Mann-Whitney U test as appropriate. The Wilcoxon signed-rank test was used to assess differences between time points within a single group. #P < .05 vs controls; +P < .05 vs baseline.
Anticoagulant effects of edoxaban in healthy volunteers and patients with cirrhosis. (A) Endogenous thrombin potential (ETP), (B) D-dimer levels, and (C) edoxaban-calibrated anti-Xa levels at baseline and in samples taken at 3 time points during administration of 60 mg edoxaban once daily to 16 patients with cirrhosis and 16 healthy controls. Horizontal lines represent medians. Values in patients and controls at a single time point were compared using Student t test or Mann-Whitney U test as appropriate. The Wilcoxon signed-rank test was used to assess differences between time points within a single group. #P < .05 vs controls; +P < .05 vs baseline.
This is the first study to monitor the effect of repeated exposure to edoxaban in a therapeutic dose in patients with cirrhosis. We chose to study a 7-day exposure time to ensure we would reach steady state, which is achieved after 3 days of dosing in healthy individuals. We found that edoxaban reduces ex vivo hemostatic potential and in vivo activation of coagulation less efficiently in patients compared with controls, despite similar plasma levels. Specifically, the relative decrease of the ETP was lower in patients compared with controls. In addition, the absolute on-drug ETP level was twofold higher in patients, suggesting insufficient anticoagulant activity in patients. Indeed, D-dimer levels clearly decreased over time in controls, but remained similar in patients. These results are in line with in vitro studies that showed Xa-inhibiting DOACs to have decreased anticoagulant effects in plasma from patients with cirrhosis.10,11 However, although we find evidence of a less effective downregulation of coagulation by edoxaban in patients with cirrhosis, clinical studies are required to assess whether this translates to a less effective antithrombotic effect in patients in both a prophylactic and a treatment setting.
Our data show that edoxaban accumulation resulting from decreased clearance does not occur in patients with mild cirrhosis. Whether drug accumulation occurs in patients with more advanced cirrhosis or in patients with cirrhosis combined with poor renal function requires further study. DOACs are cleared in part by metabolic inactivation in the liver, and in part by renal excretion. The route of elimination of edoxaban is ∼50% hepatic and 50% renal, which is roughly comparable to that of rivaroxaban (65 vs 35%) and apixaban (75 vs 25%), whereas dabigatran is cleared primarily by the kidneys (20% vs 80%). Future studies will be required to examine whether the slightly increased role of the liver in clearing rivaroxaban and apixaban results in drug accumulation at lower levels of hepatic failure. Based on our data, plasma levels of edoxaban should be as high as or perhaps somewhat higher in patients with cirrhosis compared with patients with intact liver function to obtain optimal anticoagulant effects. Although therapeutic drug levels of edoxaban have not yet been firmly established, peak levels between 120 and 250 ng/mL have been found in patients considered to be adequately treated with 60 mg once daily.12 Because edoxaban clearance may change when patients develop worsening liver disease, we propose to monitor drug levels in patients with cirrhosis using edoxaban-calibrated anti-Xa assays, which previously has been shown to be suitable for DOAC monitoring of patients with cirrhosis3 to obtain values considered therapeutic or slightly supratherapeutic in the general population.
In published studies, Xa-directed DOACs dosing tends to be conservative with reduced or low doses in a large proportion of patients.5-8 Also, in the ongoing CIRROXABAN trial (clinicaltrials.gov NCT02643212), which is a placebo-controlled trial on rivaroxaban in cirrhosis, a reduced dose was chosen. We have only studied patients with mild cirrhosis, and future studies should explore pharmacokinetics and pharmacodynamics of DOACs in patients with more advanced disease that are at increased risk for both thrombotic and bleeding complications. Nevertheless, given the hypercoagulable state of patients with cirrhosis of all severities,13 our results argue for a reevaluation of the conservative dosing regimens that have been used so far. However, although more stringent anticoagulation might lead to a better efficacy, it might also increase bleeding risk in these patients with a known complex and vulnerable hemostatic status.1,2
Taken together, edoxaban plasma levels are similar between patients with cirrhosis and healthy controls after 7 days of treatment. However, edoxaban less efficiently reduces ex vivo hemostatic potential and in vivo activation of coagulation. These data suggest that reduced dosing is not necessary and would lead to undertreatment, which is not desirable in these already hypercoagulable patients. Whether dose escalations are warranted requires further study.
For original data, please contact j.a.lisman@umcg.nl.
Acknowledgment
This investigator-initiated study was financially support by Daiichi Sankyo Europe.
Authorship
Contribution: S.B. undertook study design and patient inclusion, interpreted data, and wrote the manuscript; T.S. undertook data interpretation and revised the manuscript; H.B. interpreted the data and revised the manuscript; J.A. undertook laboratory studies, interpreted data, and revised the manuscript; T.L. undertook study design, interpreted data, and wrote the manuscript; and P.W.K. undertook study design, interpreted data, and revised the manuscript.
Conflict-of-interest disclosure: P.W.K. received an unrestricted educational grant for this study from Daiichi Sankyo Europe. The remaining authors declare no competing financial interests.
Correspondence: Ton Lisman, University Medical Center Groningen, Department of Surgery, BA33, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; e-mail: j.a.lisman@umcg.nl.
REFERENCES
Author notes
T.L. and P.W.K. are joint senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal