In this issue of Blood, Kornblit et al report on their multicenter phase 2 clinical trial (NCT01251575) investigating a new way of undertaking HLA mismatched transplantation with results that suggest a significantly improved safety profile and generally low disease relapse.1 This protocol seems to be a promising and viable new clinical option for many patients who might not otherwise receive a transplant (see figure).
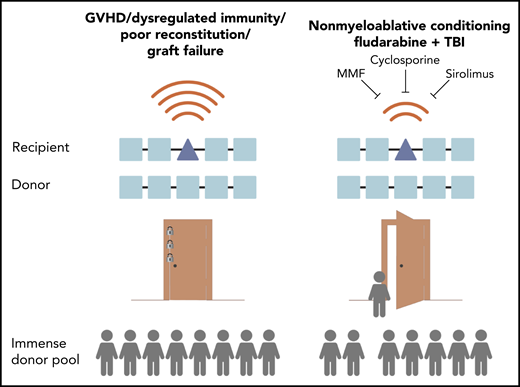
Hematopoietic stem cell transplantation between donors and recipients mismatched on 1 or more HLA alleles has historically been high risk because of complications including GVHD and dysregulated immunity. Studies of donor pools show that if HLA mismatch transplantation were safer, the vast majority of patients could receive a transplant. The Kornblit et al study of nonmyeloablative conditioning with triple-agent immunosuppression in a phase 2 clinical trial shows an improved safety profile that suggests a new option to allow more patients to receive a transplant. MMF, mycofenolate mofetil; TBI, total body irradiation.
Hematopoietic stem cell transplantation between donors and recipients mismatched on 1 or more HLA alleles has historically been high risk because of complications including GVHD and dysregulated immunity. Studies of donor pools show that if HLA mismatch transplantation were safer, the vast majority of patients could receive a transplant. The Kornblit et al study of nonmyeloablative conditioning with triple-agent immunosuppression in a phase 2 clinical trial shows an improved safety profile that suggests a new option to allow more patients to receive a transplant. MMF, mycofenolate mofetil; TBI, total body irradiation.
Ideally, every patient in need of a potentially curative hematopoietic stem cell transplantation would have a donor who is genetically matched to their HLAs. This has been the safest way to perform a transplantation because recipient immune reconstitution is generally healthier and there are fewer complications.2
Unfortunately, in reality most patients in need of a transplantation do not have suitable HLA-matched related siblings, and despite the impressive work of the National Marrow Donor Program, suitable HLA-matched unrelated donors cannot always be found. This is especially true for interracial or racial minority patients for whom the chances of finding a match are low—between 0% and 50%.2 Many of these patients can receive a transplant by using cord blood or blood from family members who are half matched (haploidentical). New protocols that make use of these alternative donors have developed rapidly over the past decade and have better safety and efficacy.
Nonetheless, there are still patients whose only option is to undergo transplantation from donors who are mismatched on 1 or more HLAs. HLA mismatched transplantation has been historically fraught with a much higher and even prohibitive rate of complications. However, a few clinical studies have suggested ways in which HLA mismatched transplantation might be done more safely, and in a way that is comparable to HLA matched transplantation.3,4 Evaluation of the unrelated donor pool has shown that if HLA mismatched transplantation were safe there would be a large untapped pool of donors available.2
The success of advances in unrelated donor matching and alternative donor transplantation means that fewer patients need to undergo transplantation with HLA mismatched unrelated donors, and it also means that accrual to trials can be low, which limits the size and design of trials that can be practically implemented. Kornblit et al report a well-executed trial involving 4 clinical sites. They compared their outcomes with recent historical results at these institutions which were that 69% of patients had grade 2 or greater acute graft-versus-host disease (aGVHD), a complication in which the donor immune system attacks the recipient’s body; they also found a nonrelapse mortality (NRM) of 47% at 2 years.
Relatively few specific HLA mismatched transplantations are considered permissive, in that the differences in the HLAs that are specifically mismatched are less immunogenic and equivalent to HLA matches, so major efforts have been undertaken to find these combinations and use them.5,6 However, the 77 patients enrolled in the Kornblit et al trial had nonpermissive mismatches.
These patients received fludarabine and total body irradiation as transplant conditioning and a triplet immunosuppression treatment that included mycofenolate mofetil, tacrolimus, and sirolimus. The conditioning used in the Kornblit et al trial is nonmyeloablative, meaning that it is among the least intensive regimens usually reserved for older and sicker patients, and patients would have hematologic recovery if donor cells and immunosuppression were not given then. In other clinical settings with more intensive conditioning, the combination of sirolimus and tacrolimus can increase toxicity. However, in the Kornblit et al trial, using all these agents together resulted in a significantly reduced aGVHD rate of 36% and a significantly lower NRM of 18% compared with historical controls. Given the trial participants ages and comorbidities, this would be a reasonable outcome even for HLA matched transplantation.
Although other groups have reported outcomes in HLA mismatch transplantation similar to those in HLA matched transplantation using nonmyeloablative conditioning,3,4 the trial by Kornblit et al stands out for several reasons. First, the other protocols all used anti-thymocyte globulin (ATG) as part of the immunosuppression protocol, but this study suggests that ATG may not be necessary, perhaps because lymphodepletion is achieved by fludarabine. ATG is associated with a number of infectious disease complications. Second, the relapse and progression observed in the trial could be lower than those in other approaches, although there are many caveats to making direct comparisons.
The work of Kornblit et al also highlights that effectively managing nonmyeloablative transplantation may take years as opposed to months. For example, they observed that the rate of chronic GVHD (cGVHD) was initially low but then climbed to rates near those seen historically by 4 years. In the other approaches using ATG, the incidence rates for cGVHD may be lower over time, which suggests the possibility of using minimal-effective-dose immunosuppression or other approaches that might be worth testing with this new strategy.3,4 Likewise, although patients may relapse after nonmyeloablative transplantation, posttransplant treatment can stimulate donor graft-versus-leukemia (GVL) and restore durable remissions, a fact that makes comparisons of relapse and disease control more complicated.
All these promising HLA mismatched transplantation strategies rely on radiation as part of conditioning. Radiation has immune-modulatory effects by eliciting apoptotic pathways and is often associated with immune tolerance. Whether radiation is needed scientifically to allow HLA mismatch transplantation has not been clinically tested. There are also newer strategies that have been successfully used preclinically in major histocompatibility mismatch transplantation, such as the use of immunoregulatory TR1 cells which is now being tested in HLA mismatched transplantation for full-intensity conditioning.6
Although HLA mismatched transplantation is often framed as an option of last resort, studies like that of Kornblit et al suggest that this may not be the case in the future, especially because there have been significant improvements in finding donors for all those who need transplantation.2 Importantly, genetic mismatches could potentially be exploited to enhance GVL effects, as has been suggested for sex mismatch in the nonmyeloablative setting.7 Likewise, a donor in a large HLA mismatch pool could be chosen on the basis of other factors that could enhance GVL, such as killer-cell immunoglobulin-like receptors.8 More clinical trials of the same high caliber as the Kornblit et al trial are needed to advance the practical implementation of HLA mismatch transplantation so that every patient in need can receive a transplant.
Conflict-of-interest disclosure: E.M. serves as scientific advisor and is an equity holder in GigaGen Inc. and Triursus Therapeutics, and he has a sponsored research agreement with Orca Biosystems.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal