In this issue of Blood, Nadeu et al provide new data on the origin and pathogenesis of mantle cell lymphoma (MCL), showing how genetic and epigenetic features of the tumor relate to its clinical behavior.1
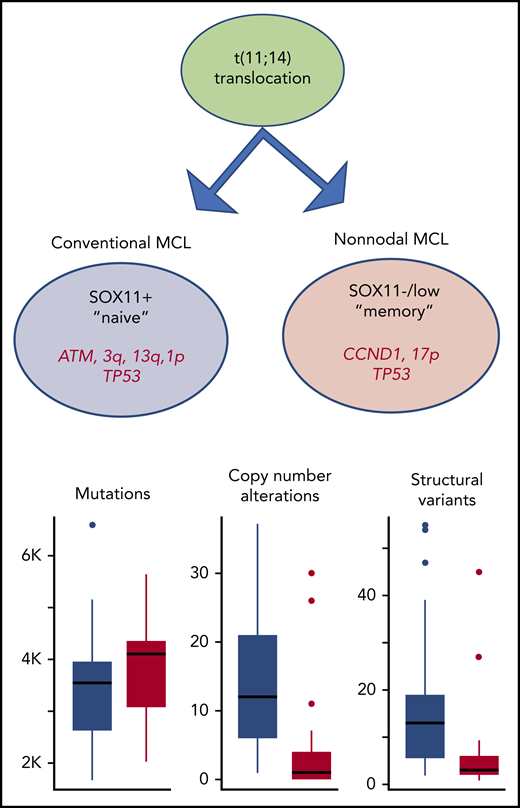
The primary genetic event in MCL is the t(11;14)(q13;q32) CCND1/IGH translocation (in green). The cell carrying the cyclin D1 translocation can either follow a path similar to that of naïve B cells with unmutated immunoglobulin genes (conventional MCL, in blue). These MCL tumors mostly express SOX11. Approximately half of the tumors have ATM aberrations and, as shown in the article by Nadeu et al, they are genetically unstable with a high number of copy number alterations and structural variants. Alternatively, the cells with cyclin D1 translocations show evidence of a germinal center experience with mutated immunoglobulin genes and low expression of SOX11 (nonnodal MCL, in red). Nadeu et al show that the nonnodal MCL tumors are characterized by fewer copy number alterations and structural variants with the exception of del17p/TP53 which is enriched. Nonnodal MCL cells also carry a higher frequency of CCND1 somatic mutations.
The primary genetic event in MCL is the t(11;14)(q13;q32) CCND1/IGH translocation (in green). The cell carrying the cyclin D1 translocation can either follow a path similar to that of naïve B cells with unmutated immunoglobulin genes (conventional MCL, in blue). These MCL tumors mostly express SOX11. Approximately half of the tumors have ATM aberrations and, as shown in the article by Nadeu et al, they are genetically unstable with a high number of copy number alterations and structural variants. Alternatively, the cells with cyclin D1 translocations show evidence of a germinal center experience with mutated immunoglobulin genes and low expression of SOX11 (nonnodal MCL, in red). Nadeu et al show that the nonnodal MCL tumors are characterized by fewer copy number alterations and structural variants with the exception of del17p/TP53 which is enriched. Nonnodal MCL cells also carry a higher frequency of CCND1 somatic mutations.
The genetic hallmark of MCL is the rearrangement of the cyclin D1 gene. Most MCL are similar to naïve B cells with unmutated immunoglobulin genes. At diagnosis, there is lymph node involvement and often widespread disease involving the bone marrow and gastrointestinal tract, so upfront treatment is usually required. But for a minority of patients with conventional MCL, treatment may be deferred for several years.2,3 Importantly, there are patients with nonnodal leukemic disease who have MCL with mutated immunoglobulin genes (a sign of follicle germinal center history), and their disease has clearly indolent behavior, which is now recognized in the World Health Organization classification of lymphoid malignancies.4-6 Ten years ago, Fernàndez et al6 suggested that conventional and nonnodal MCL could be discriminated on the basis of a gene expression signature such as low SOX11 expression in patients with nonnodal disease.
What determines the difference in biology and clinical presentation between conventional and nonnodal MCL? Are there factors that might explain why MCL is sometimes indolent? In their study, Nadeu et al used a gene expression signature that included SOX11 (or, in a few cases, SOX11 immunohistochemistry) to classify the disease into either conventional or nonnodal MCL. The lymphomas were then extensively characterized by whole-genome, whole-exome, transcriptome, and methylation profiling, which revealed interesting similarities and differences between the 2 subtypes.
The study by Nadeu et al is important for several reasons. First, they show that the underlying primary genetic event, the cyclin D1 rearrangement, occurred by similar mechanisms in the 2 MCL subtypes. The translocation of the cyclin D1 gene occurred mostly during V(D)J rearrangement in precursor B cells. However, in a few instances (including both conventional and nonnodal MCL), the rearrangement seemed to arise in mature B cells. Second, the authors show that additional genetic alterations, particularly copy number alterations, were significantly more frequent in conventional MCL and that this high genomic complexity, rather than the number of gene mutations, was associated with patient outcome. Specifically, DNA breakage-fusion bridge cycles were seen only in conventional MCL. DNA breakage-fusion bridge cycles are caused by the loss of telomeres at the end regions of chromosomes, which leads to fusion of the sister chromatids that subsequently break apart during cell division and result in more breakage-fusion bridges and ongoing genetic instability.7 Moreover, gene mutations also differed in the 2 subtypes. Interestingly, ATM was inactivated only in conventional MCL, whereas loss of 17p (TP53) and TP53 mutations were seen in both subtypes but were enriched in nonnodal MCL (see figure).
This study provides important new insights into the pathogenesis of MCL but also points to several unresolved questions. The relative frequency of nonnodal MCL compared with conventional MCL is currently not known because nonnodal MCL may have an unconventional immune phenotype and can therefore slip under the radar and be misdiagnosed as another type of mature B-cell lymphoma. ATM inactivation is a feature of conventional MCL and might contribute to the high genomic instability. Intriguingly, TP53 aberrations are enriched in nonnodal MCL in spite of their higher genomic stability and more indolent disease course. It could be hypothesized that there is an association with lack of SOX11 expression, because p53 alterations are enriched not only in nonnodal MCL, as shown by Nadeu et al, but are also seen in the small proportion of patients with conventional MCL with low SOX11 expression.3,8,9 The underlying mechanisms for this peculiar association between SOX11 negativity and TP53 aberrations in MCL are not known and need to be studied. Finally, if the underlying primary genetic event is similar in both subtypes of MCL, then what guides the further development into either conventional or nonnodal MCL? Which signals make the MCL precursor cell decide whether to enter the germinal center or not?
Conflict-of-interest disclosure: The author declares no competing financial interests.