Key Points
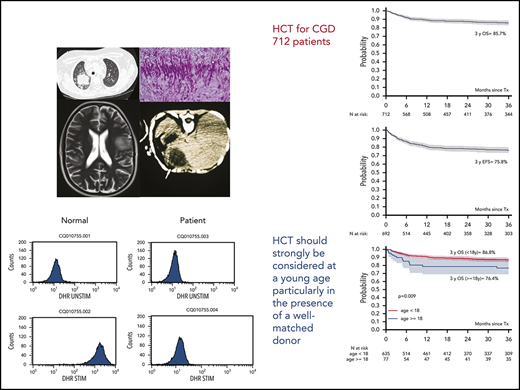
An excellent outcome was obtained after allo-HCT in 712 patients with CGD, with a low incidence of graft failure and mortality.
HCT for CGD should be strongly considered in young patients, particularly in the presence of a well-matched donor.
Abstract
Chronic granulomatous disease (CGD) is a primary immunodeficiency resulting in life-threatening infections and inflammatory complications. Allogeneic hematopoietic cell transplantation (allo-HCT) can cure the disease, but the indication to transplant remains controversial. We performed a retrospective multicenter study of 712 patients with CGD who underwent allo-HCT transplantation from March 1993 through December 2018. We studied 635 children (aged <18 years) and 77 adults. Median follow-up was 45 months. Median age at transplantation was 7 years (range, 0.1-48.6). Kaplan-Meier estimates of overall survival (OS) and event-free survival (EFS) at 3 years were 85.7% and 75.8%, respectively. In multivariate analysis, older age was associated with reduced survival and increased chronic graft-versus-host disease. Nevertheless, OS and EFS at 3 years for patients ≥18 years were 76% and 69%, respectively. Use of 1-antigen-mismatched donors was associated with reduced OS and EFS . No significant difference was found in OS, but a significantly reduced EFS was noted in the small group of patients who received a transplant from a donor with a >1 antigen mismatch. Choice of conditioning regimen did not influence OS or EFS. In summary, we report an excellent outcome after allo-HCT in CGD, with low incidence of graft failure and mortality in all ages. Older patients and recipients of 1-antigen-mismatched grafts had a less favorable outcome. Transplantation should be strongly considered at a younger age and particularly in the presence of a well-matched donor.
Introduction
Chronic granulomatous disease (CGD) is an inherited primary immunodeficiency caused by mutations in genes encoding subunits of the nicotinamide adenine dinucleotide phosphate oxidase complex. The impaired production of the superoxide anion and other reactive oxygen intermediates by neutrophils, monocytes, and macrophages leads to impaired microbe killing, life-threatening bacterial and fungal infections, and immune dysregulation and hyperinflammation.1 Inheritance of CGD can be X-linked or autosomal recessive (AR), with an incidence that varies from 1 in 200 000 live births in the United States and Europe to 1 in 70 000 in the Israeli Arab population.2
Despite the use of prophylactic antibacterial and antifungal medications and improved management of infections and inflammatory complications, mortality remains high, with registry studies reporting a survival of 50% to 55% through the fourth decade of life.3-5 Moreover, surviving patients often experience growth failure and severe organ dysfunction, such as inflammatory lung disease, chronic colitis, and kidney failure. These comorbidities constitute a significant health burden, causing reduced school and professional performance and poor quality of life.6,7
Allogeneic hematopoietic cell transplantation (allo-HCT) can cure CGD, with resolution of infections and inflammatory complications.8,9 Nevertheless, indication to treat with HCT in CGD, especially when transplants are from unrelated or mismatched donors, is reasonably recent, and the limited published data mostly describe the pediatric population.
The Inborn Errors Working Party (IEWP) of the European Society for Blood and Marrow Transplantation (EBMT) conducted a multicenter retrospective analysis of data from 635 children (aged <18 years) and 77 adults (aged ≥18 years) who were affected by CGD and underwent allogeneic hematopoietic stem cell transplantation between March 1993 and December 2018.
Patients and methods
Data source
The registry of the EBMT provided demographic, laboratory, and clinical data, and an additional specific study questionnaire was completed by the study centers. This study was approved by the review board of the IEWP of the EBMT (study number, 7427005). The EBMT is a voluntary working group, including >500 transplantation units that are requested to report all consecutive allo-HCTs and follow-up visits once each year. Audits are routinely performed to ensure the accuracy of the data. In accordance with the Declaration of Helsinki, patients give informed consent for data entry into the EBMT registry database and for its use in analyses.
Study participants and transplantation procedures
All patients who underwent allo-HCT for CGD in any of the EBMT centers from March 1993 through December 2018 were eligible for this study. There were 759 patients in the EBMT database, but 47 had no follow-up information and were excluded from the analysis.
We recorded data on genetic pattern of inheritance (AR vs X-linked CGD), the presence of comorbidities before transplantation that were deemed to be medically relevant by the investigators (infections, chronic colitis, malnutrition, liver derangement, and renal derangement), age at transplantation, donor type, degree of HLA match, stem cell source, conditioning regimen, and graft-versus-host disease (GVHD) prophylaxis.
HLA compatibility with an adult related or unrelated donor was defined by high- to medium-resolution typing for HLA-A, B, -C, -DR, and -DQ loci. For an umbilical cord blood transplant, HLA compatibility requirements followed the current practice of antigen level typing for HLA-A and -B and allele level typing for HLA-DRB1.
Myeloid recovery was defined as the first of 3 consecutive days with an absolute neutrophil count >0.5 × 109/L, whereas platelet recovery was defined as an unsupported platelet count >20 × 109/L. Graft rejection was defined as the presence of donor DNA in peripheral blood <5%.
Grading of acute and chronic GVHD (aGVHD and cGVHD, respectively) was performed according to the Seattle criteria.10,11
Statistical analysis
Patient and transplant characteristics were expressed as the number and percentage of the group for categorical variables and median with ranges for continuous variables. The time origin for time-to-event analysis was the first allo-HCT, and patients alive without an event after transplant were censored at the last follow-up or the time of data extraction.
The primary end points were overall survival (OS), where an event was defined as death of any cause, and event-free survival (EFS), where an event was defined as graft failure (GF) or death, whichever happened first. Secondary end points were GF and aGVHD. Data on infection, colitis, and autoimmune complications were insufficient to include these events as secondary end points. GF and non-GF mortality were analyzed as competing risks.
Univariate analyses (UVAs) were performed with the Kaplan-Meier method, and survival curves were compared by using the log-rank test for OS and EFS, whereas for competing risks events, cumulative incidence curves were compared by using Gray’s test. Multivariate analyses (MVAs) were performed using the Cox proportional hazards model for OS and EFS and the cause-specific Cox model for GF, non-GF mortality, acute GVHD, and chronic GVHD, to identify independent factors that are prognostic of the outcomes mentioned. Variables included in the MVA were age at transplantation, presence of colitis, conditioning regimen, donor type, and stem cell source. No statistical variable selection was performed, and the inclusion of these variables was discussed and agreed to by an expert panel. All statistical analyses were performed with R, version 3.5.2 (packages prodlim, survival, and maxstat), and RStudio, version 1.1.463. A value of P < .05 indicated statistically significant results.
Results
Characteristics of patients and transplants
This analysis included 712 patients affected by CGD who underwent allo-HCT transplantation across 101 EBMT Centers. A detailed description of the patients and their transplants is shown in Table 1.
Patient and transplant characteristics
| . | Overall . | Age <18 y . | Age ≥18 y . |
|---|---|---|---|
| n (%) . | n (%) . | n (%) . | |
| Study participants | 712 (100) | 635 (89) | 77 (11) |
| Median age (y), range (min, max) | 7.08 (0.190-48.6) | 5.73 (0.190-18.0) | 21.9 (18.0-48.6) |
| Sex | |||
| Male | 617 (86.7) | 551 (86.8) | 66 (85.7) |
| Female | 93 (13.1) | 82 (12.9) | 11 (14.3) |
| Missing data | 2 (0.3) | 2 (0.3) | 0 (0) |
| Pattern of inheritance | |||
| Autosomal recessive CGD | 104 (14.6) | 90 (14.2) | 14 (18.2) |
| X-linked CGD | 309 (43.4) | 278 (43.8) | 31 (40.3) |
| Missing data | 299 (42.0) | 267 (42.0) | 32 (41.6) |
| Comorbidities before transplantation | |||
| Infections | |||
| No | 160 (22.5) | 132 (20.8) | 28 (36.4) |
| Yes | 339 (47.6) | 309 (48.7) | 30 (39.0) |
| Not evaluated | 0 (0) | 0 (0) | 0 (0) |
| Missing data | 213 (29.9) | 194 (30.6) | 19 (24.7) |
| Liver involvement | |||
| Absent | 411 (57.7) | 374 (58.9) | 37 (48.1) |
| Present | 41 (5.8) | 37 (5.8) | 4 (5.2) |
| Missing data | 260 (36.5) | 224 (35.3) | 36 (46.8) |
| Colitis | |||
| Absent | 346 (48.6) | 320 (50.4) | 26 (33.8) |
| Present | 108 (15.2) | 91 (14.3) | 17 (22.1) |
| Missing data | 258 (36.2) | 224 (35.3) | 34 (44.2) |
| Malnutrition | |||
| Absent | 338 (47.5) | 306 (48.2) | 32 (41.6) |
| Present | 112 (15.7) | 103 (16.2) | 9 (11.7) |
| Missing data | 262 (36.8) | 226 (35.6) | 36 (46.8) |
| Renal involvement | |||
| Absent | 427 (60.0) | 391 (61.6) | 36 (46.8) |
| Present | 25 (3.5) | 20 (3.1) | 5 (6.5) |
| Missing data | 260 (36.5) | 224 (35.3) | 36 (46.8) |
| Conditioning regimen | |||
| Busulfan, fludarabine | 323 (45.4) | 270 (42.5) | 53 (68.8) |
| Busulfan, cyclophosphamide | 113 (15.9) | 112 (17.6) | 1 (1.3) |
| Treosulfan, fludarabine | 89 (12.5) | 84 (13.2) | 5 (6.5) |
| Treosulfan, fludarabine, thiotepa | 58 (8.1) | 54 (8.5) | 4 (5.2) |
| Other combinations | 111 (15.6) | 98 (15.5) | 13 (16.9) |
| Missing data | 18 (2.5) | 17 (2.7) | 1 (1.3) |
| In vivo T-cell depletion | |||
| ATG | 264 (37.1) | 230 (36.2) | 34 (44.2) |
| Alemtuzumab | 265 (37.2) | 240 (37.8) | 25 (32.5) |
| None | 178 (25) | 161 (25.4) | 17 (22.1) |
| Other | 5 (0.7) | 4 (0.6) | 1 (1.3) |
| In vitro T-cell depletion | |||
| Absent (no mismatch) | 469 (65.9) | 414 (65) | 55 (71.4) |
| Absent (1-Ag mismatch) | 85 (12) | 69 (11) | 16 (20.7) |
| Absent (>1-Ag mismatch) | 10 (1.4) | 9 (1.5) | 1 (1.4) |
| Present (no mismatch) | 35 (5) | 33 (5) | 2 (2.5) |
| Present (1-Ag mismatch) | 12 (1.6) | 12 (2) | 0 (0) |
| Present (>1-Ag mismatch) | 16 (2.2) | 16 (2.5) | 0 (0) |
| Missing data | 85 (11.9) | 82 (13) | 3 (4) |
| Donor type | |||
| MFD | 257 (36.1) | 233 (36.7) | 24 (31.2) |
| MUD* | 290 (40.7) | 258 (40.6) | 32 (41.6) |
| MMFD | 36 (5.1) | 35 (5.5) | 1 (1.3) |
| MMUD* | 107 (15.0) | 90 (14.2) | 17 (22.1) |
| Other/missing data | 22 (3.1) | 19 (3) | 3 (3.9) |
| HLA matching | |||
| No mismatch (PB, BM10/10 or CB 6/6) | 547 (76.8) | 491 (77.3) | 56 (72.7) |
| 1 Ag mismatch (PB,BM 9/10 or CB 5/6) | 105 (14.7) | 88 (13.9) | 17 (22.1) |
| >1 Ag mismatch (PB,BM <9/10 or CB <5/6) | 29 (4.1) | 28 (4.4) | 1 (1.3) |
| Missing data | 31 (4.4) | 28 (4.4) | 3 (3.9) |
| Stem cell source | |||
| Bone marrow | 468 (65.7) | 427 (67.2) | 41 (53.2) |
| Peripheral blood | 200 (28.1) | 166 (26.1) | 34 (44.2) |
| Umbilical cord blood | 30 (4.2) | 29 (4.6) | 1 (1.3) |
| Other/missing data | 14 (0.5) | 13 (2.1) | 1 (1.3) |
| Year of transplantation | |||
| 1993-2005 | 92 (13) | 81 (12.7) | 11 (14) |
| 2006-2018 | 620 (87) | 554 (87.3) | 66 (86) |
| . | Overall . | Age <18 y . | Age ≥18 y . |
|---|---|---|---|
| n (%) . | n (%) . | n (%) . | |
| Study participants | 712 (100) | 635 (89) | 77 (11) |
| Median age (y), range (min, max) | 7.08 (0.190-48.6) | 5.73 (0.190-18.0) | 21.9 (18.0-48.6) |
| Sex | |||
| Male | 617 (86.7) | 551 (86.8) | 66 (85.7) |
| Female | 93 (13.1) | 82 (12.9) | 11 (14.3) |
| Missing data | 2 (0.3) | 2 (0.3) | 0 (0) |
| Pattern of inheritance | |||
| Autosomal recessive CGD | 104 (14.6) | 90 (14.2) | 14 (18.2) |
| X-linked CGD | 309 (43.4) | 278 (43.8) | 31 (40.3) |
| Missing data | 299 (42.0) | 267 (42.0) | 32 (41.6) |
| Comorbidities before transplantation | |||
| Infections | |||
| No | 160 (22.5) | 132 (20.8) | 28 (36.4) |
| Yes | 339 (47.6) | 309 (48.7) | 30 (39.0) |
| Not evaluated | 0 (0) | 0 (0) | 0 (0) |
| Missing data | 213 (29.9) | 194 (30.6) | 19 (24.7) |
| Liver involvement | |||
| Absent | 411 (57.7) | 374 (58.9) | 37 (48.1) |
| Present | 41 (5.8) | 37 (5.8) | 4 (5.2) |
| Missing data | 260 (36.5) | 224 (35.3) | 36 (46.8) |
| Colitis | |||
| Absent | 346 (48.6) | 320 (50.4) | 26 (33.8) |
| Present | 108 (15.2) | 91 (14.3) | 17 (22.1) |
| Missing data | 258 (36.2) | 224 (35.3) | 34 (44.2) |
| Malnutrition | |||
| Absent | 338 (47.5) | 306 (48.2) | 32 (41.6) |
| Present | 112 (15.7) | 103 (16.2) | 9 (11.7) |
| Missing data | 262 (36.8) | 226 (35.6) | 36 (46.8) |
| Renal involvement | |||
| Absent | 427 (60.0) | 391 (61.6) | 36 (46.8) |
| Present | 25 (3.5) | 20 (3.1) | 5 (6.5) |
| Missing data | 260 (36.5) | 224 (35.3) | 36 (46.8) |
| Conditioning regimen | |||
| Busulfan, fludarabine | 323 (45.4) | 270 (42.5) | 53 (68.8) |
| Busulfan, cyclophosphamide | 113 (15.9) | 112 (17.6) | 1 (1.3) |
| Treosulfan, fludarabine | 89 (12.5) | 84 (13.2) | 5 (6.5) |
| Treosulfan, fludarabine, thiotepa | 58 (8.1) | 54 (8.5) | 4 (5.2) |
| Other combinations | 111 (15.6) | 98 (15.5) | 13 (16.9) |
| Missing data | 18 (2.5) | 17 (2.7) | 1 (1.3) |
| In vivo T-cell depletion | |||
| ATG | 264 (37.1) | 230 (36.2) | 34 (44.2) |
| Alemtuzumab | 265 (37.2) | 240 (37.8) | 25 (32.5) |
| None | 178 (25) | 161 (25.4) | 17 (22.1) |
| Other | 5 (0.7) | 4 (0.6) | 1 (1.3) |
| In vitro T-cell depletion | |||
| Absent (no mismatch) | 469 (65.9) | 414 (65) | 55 (71.4) |
| Absent (1-Ag mismatch) | 85 (12) | 69 (11) | 16 (20.7) |
| Absent (>1-Ag mismatch) | 10 (1.4) | 9 (1.5) | 1 (1.4) |
| Present (no mismatch) | 35 (5) | 33 (5) | 2 (2.5) |
| Present (1-Ag mismatch) | 12 (1.6) | 12 (2) | 0 (0) |
| Present (>1-Ag mismatch) | 16 (2.2) | 16 (2.5) | 0 (0) |
| Missing data | 85 (11.9) | 82 (13) | 3 (4) |
| Donor type | |||
| MFD | 257 (36.1) | 233 (36.7) | 24 (31.2) |
| MUD* | 290 (40.7) | 258 (40.6) | 32 (41.6) |
| MMFD | 36 (5.1) | 35 (5.5) | 1 (1.3) |
| MMUD* | 107 (15.0) | 90 (14.2) | 17 (22.1) |
| Other/missing data | 22 (3.1) | 19 (3) | 3 (3.9) |
| HLA matching | |||
| No mismatch (PB, BM10/10 or CB 6/6) | 547 (76.8) | 491 (77.3) | 56 (72.7) |
| 1 Ag mismatch (PB,BM 9/10 or CB 5/6) | 105 (14.7) | 88 (13.9) | 17 (22.1) |
| >1 Ag mismatch (PB,BM <9/10 or CB <5/6) | 29 (4.1) | 28 (4.4) | 1 (1.3) |
| Missing data | 31 (4.4) | 28 (4.4) | 3 (3.9) |
| Stem cell source | |||
| Bone marrow | 468 (65.7) | 427 (67.2) | 41 (53.2) |
| Peripheral blood | 200 (28.1) | 166 (26.1) | 34 (44.2) |
| Umbilical cord blood | 30 (4.2) | 29 (4.6) | 1 (1.3) |
| Other/missing data | 14 (0.5) | 13 (2.1) | 1 (1.3) |
| Year of transplantation | |||
| 1993-2005 | 92 (13) | 81 (12.7) | 11 (14) |
| 2006-2018 | 620 (87) | 554 (87.3) | 66 (86) |
1-Ag MM, 1-antigen-mismatched donor; BM, bone marrow; CB, cord blood; MMFD, mismatched family donor; MMUD, mismatched unrelated donor; PB, peripheral blood.
Full information on HLA typing in MUD/MMUD transplants was available in 354 of 397 cases (high resolution, 10 alleles in 252 of 354; low resolution, <10 alleles in 102 of 354).
We studied 635 children (aged <18 years) and 77 adults (aged ≥18 years). The pattern of inheritance was X-linked or AR in 309 of 413 (75%) and 104 of 413 (25%) evaluable patients, respectively. The median age at transplantation was 7 years (range, 0.1-48.6). The disease burden was significant: pretransplant infections, chronic colitis, liver derangement, and renal impairment were documented in 339 of 499 (68%), 108 of 454 (24%), 41 of 452 (9%), and 25 of 452 (5%) evaluable patients, respectively.
The most common conditioning regimen was busulfan and fludarabine (45.5%), followed by busulfan and cyclophosphamide (15.9%); treosulfan and fludarabine (12.5%); and treosulfan, fludarabine, and thiotepa (8.1%), reflecting the IEWP guidelines in effect during the study period.12 Patients received grafts from matched related or unrelated donors in 547 of 681 (80%) cases, whereas 134 of 681 (20%) patients received grafts from mismatched donors. The median follow-up was 45 months (interquartile range, 17.64-82.79). The mean age of patients in the matched family donor group was 8.44 years, whereas the mean age in the mismatched or unrelated donor group was 9.35 years. Older patients were more likely to receive mismatched or unrelated donor transplants, but the difference was not statistically significant (odds ratio, 1.02; P = .11). Eighty-seven percent of patients underwent transplantation after 2006.
Overall survival
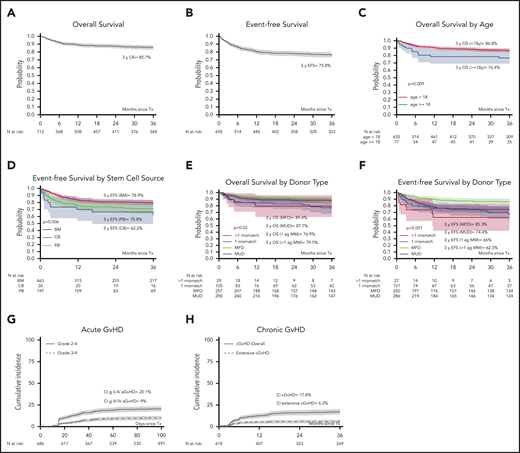
Six hundred and twenty patients were alive at last follow-up, giving a 3-year OS of 85.7% (95% confidence interval [CI], 82.8-88.5; Figure 1A).
Probabilities of OS, EFS, and cumulative incidence of aGVHD and cGBHD in 712 patients with CDG. OS at 3 years was 85.7% (A) and EFS at 3 years was 75.8% (B) in 712 children and adults with CGD who underwent allo-HCT. (C) OS at 3 years was higher in 635 children aged <8 years (86.8%), compared with 77 adults aged ≥18 years (76.4%). In UVA, (D) EFS at 3 years was influenced by the stem cell source (BM, 78.9%; PB, 70.8%; and CB, 62.2%); (E) OS at 3 years was influenced by donor type (MFD, 89.4%; MUD, 87.7%; >1 Ag MM, 79.5%; and 1 Ag MM, 76.9%); and (F) EFS at 3 years was influenced by donor type (MFD, 85.3%; MUD, 74.4%; 1 Ag MM, 66%; and >1 Ag MM, 62.3%). The cumulative incidence of (G) grades II-IV and grades III-IV aGVHD was 20.1% and 9%, respectively, and that of (H) cGVHD and extensive chronic GVHD at 3 years was 17.8% and 6.2%, respectively. 1-Ag MM, 1-antigen-mismatched donor; BM, bone marrow; CB, cord blood; PB, peripheral blood.
Probabilities of OS, EFS, and cumulative incidence of aGVHD and cGBHD in 712 patients with CDG. OS at 3 years was 85.7% (A) and EFS at 3 years was 75.8% (B) in 712 children and adults with CGD who underwent allo-HCT. (C) OS at 3 years was higher in 635 children aged <8 years (86.8%), compared with 77 adults aged ≥18 years (76.4%). In UVA, (D) EFS at 3 years was influenced by the stem cell source (BM, 78.9%; PB, 70.8%; and CB, 62.2%); (E) OS at 3 years was influenced by donor type (MFD, 89.4%; MUD, 87.7%; >1 Ag MM, 79.5%; and 1 Ag MM, 76.9%); and (F) EFS at 3 years was influenced by donor type (MFD, 85.3%; MUD, 74.4%; 1 Ag MM, 66%; and >1 Ag MM, 62.3%). The cumulative incidence of (G) grades II-IV and grades III-IV aGVHD was 20.1% and 9%, respectively, and that of (H) cGVHD and extensive chronic GVHD at 3 years was 17.8% and 6.2%, respectively. 1-Ag MM, 1-antigen-mismatched donor; BM, bone marrow; CB, cord blood; PB, peripheral blood.
Ninety-two patients died after allo-HCT of infections (42%), GVHD (33%), toxicity/organ damage (11%), and other complications (14%; Table 2).
Transplant complications and outcomes
| . | n (%) . |
|---|---|
| Status at last follow-up | |
| Alive | 620 (87) |
| Dead | 92 (13) |
| Cause of death (n = 92) | |
| Infections | 39 (42) |
| GVHD | 30 (33) |
| Toxicity/organ damage | 10 (11) |
| PTLD | 2 (2) |
| Other | 8 (9) |
| Missing data | 3 (3) |
| Donor engraftment | |
| Engraftment | 610 (88) |
| Primary GF | 13 (1.8) |
| Secondary GF | 71 (10.0) |
| Missing data | 18 (2.5) |
| Neutrophil recovery | |
| Median days after HSCT (range) | 18 (3.00, 133) |
| Missing data | 46 (6.5) |
| Platelet recovery | |
| Median days after HSCT (range) | 19 (3.00, 131) |
| Missing data | 202 (28.4) |
| aGVHD | |
| No aGVHD/grade I | 541 (76.0) |
| Grade II | 81 (11.4) |
| Grade III or IV | 64 (9.0) |
| Missing data | 26 (3.7) |
| cGVHD | |
| No cGVHD | 513 (72.1) |
| Limited | 53 (7.4) |
| Extensive | 33 (4.6) |
| Missing data | 113 (15.8) |
| . | n (%) . |
|---|---|
| Status at last follow-up | |
| Alive | 620 (87) |
| Dead | 92 (13) |
| Cause of death (n = 92) | |
| Infections | 39 (42) |
| GVHD | 30 (33) |
| Toxicity/organ damage | 10 (11) |
| PTLD | 2 (2) |
| Other | 8 (9) |
| Missing data | 3 (3) |
| Donor engraftment | |
| Engraftment | 610 (88) |
| Primary GF | 13 (1.8) |
| Secondary GF | 71 (10.0) |
| Missing data | 18 (2.5) |
| Neutrophil recovery | |
| Median days after HSCT (range) | 18 (3.00, 133) |
| Missing data | 46 (6.5) |
| Platelet recovery | |
| Median days after HSCT (range) | 19 (3.00, 131) |
| Missing data | 202 (28.4) |
| aGVHD | |
| No aGVHD/grade I | 541 (76.0) |
| Grade II | 81 (11.4) |
| Grade III or IV | 64 (9.0) |
| Missing data | 26 (3.7) |
| cGVHD | |
| No cGVHD | 513 (72.1) |
| Limited | 53 (7.4) |
| Extensive | 33 (4.6) |
| Missing data | 113 (15.8) |
Data are the number of patients (percentage of the total group), unless otherwise stated.
PTLD, posttransplant lymphoproliferative disorder.
In UVA, OS was influenced by age (P = .009), pretransplant colitis (P = .01), and donor type (P = .02; Table 3; Figure 1C,E). The genetic pattern of inheritance did not influence survival (Table 3).
Univariate analysis
| . | 3-y OS (95% CI) . | 3-y EFS (95% CI) . | 3-y GF (95% CI) . | Grade III-IV aGVHD (95% CI) . | cGVHD (95% CI) . |
|---|---|---|---|---|---|
| Age | |||||
| <18 y | 0.86 (0.83-0.89) | 0.76 (0.72-0.8) | 0.13 (0.10-0.16) | 0.08 (0.06-0.11) | 0.17 (0.13-0.20) |
| ≥18 y | 0.76 (0.66-0.86) | 0.69 (0.57 0.8) | 0.12 (0.04-0.21) | 0.10 (0.03-0.1) | 0.22 (0.11-0.33) |
| P | .009 | .2 | .74 | 066 | .40 |
| Genetics | |||||
| AR | 0.86 (0.80-0.93) | 0.77 (0.69-0.85) | 0.11 (0.05-0.17) | 0.09 (0.03-0.15) | 0.14 (0.07-0.22) |
| X-linked | 0.85 (0.81-0.89) | 0.75 (0.70-0.80) | 0.14 (0.10-0.18) | 0.08 (0.05-0.11) | 0.15 (0.11-0.20) |
| P | .9 | .7 | .39 | .66 | .65 |
| Colitis | |||||
| Present | 0.78 (0.70-0.86) | 0.72 (0.63-0.81) | 0.09 (0.03-0.14) | 0.11 (0.05-0.17) | 0.18 (0.10-0.26) |
| Absent | 0.87 (0.84-0.91) | 0.76 (0.71-0.8) | 0.15 (0.11-0.19) | 0.08 (0.05-0.11) | 0.14 (0.10-0.18) |
| P | .01 | .5 | .08 | .33 | .31 |
| Liver derangement | |||||
| Present | 0.90 (0.80-0.99) | 0.89 (0.79-0.99) | 0.05 (0-0.13) | 0.05 (0-0.11) | 0.13 (0.01-0.26) |
| Absent | 0.84 (0.81-0.88) | 0.74 (0.69-0.78) | 0.14 (0.10-0.17) | 0.08 (0.06-0.11) | 0.16 (0.12-0.19) |
| P | .4 | .04 | .11 | .38 | .52 |
| Renal derangement | |||||
| Present | 0.76 (0.59-0.92) | 0.75 (0.57-0.92) | 0.16 (0.01-0.31) | 0.08 (0-0.19) | 0.17 (0.01-0.33) |
| Absent | 0.85 (0.81-0.88) | 0.74 (0.7-0.79) | 0.14 (0.10-0.17) | 0.08 (0.05-0.10) | 0.15 (0.11-0.19) |
| P | .1 | .8 | .62 | .99 | .76 |
| Infections | |||||
| Present | 0.83 (0.79-0.88) | 0.74 (0.69-0.79) | 0.13 (0.09-0.17) | 0.08 (0.05-0.12) | 0.14 (0.10-0.18) |
| Absent | 0.87 (0.81-0.92) | 0.74 (0.67-0.81) | 0.14 (0.08-0.20) | 0.06 (0.02-0.10) | 0.16 (0.10-0.23) |
| P | .4 | .9 | .65 | .32 | .62 |
| Conditioning regimen | |||||
| Bu/cy | 0.88 (0.82-0.94) | 0.84 (0.77-0.91) | 0.03 (0.001-0.07) | 0.11 (0.05-0.17) | 0.13 (0.07-0.20) |
| Bu/flu | 0.84 (0.79-0.88) | 0.75 (0.7-0.8) | 0.13 (0.09-0.17) | 0.07 (0.04-0.09) | 0.20 (0.15-0.26) |
| Treo/flu | 0.90 (0.82-0.98) | 0.71 (0.59-0.83) | 0.22 (0.11-0.33) | 0.06 (0.01-0.12) | 0.09 (0.02-0.15) |
| Treo/flu/TT | 0.95 (0.89-1) | 0.85 (0.74-0.95) | 0.10 (0.01-0.19) | 0.09 (0.01-0.16) | 0.07 (0-0.16) |
| P | .09 | .1 | .009 | .38 | .04 |
| In vivo T-cell depletion | |||||
| ATG | 0.91 (0.85-0.97) | 0.84 (0.76-0.93) | 0.09 (0.02-0.16) | 0.05 (0.01-0.10) | 0.18 (0.09-0.27) |
| Alemtuzumab | 0.92 (0.81-1) | 0.88 (0.76-1) | 0.07 (0-0.18) | 0.03 (0-0.09) | 0.04 (0-0.12) |
| None | 0.86 (0.80-0.93) | 0.84 (0.77-0.91) | 0.02 (0-0.06) | 0.15 (0.08-0.22) | 0.16 (0.08-0.24) |
| P | .4 | .8 | .25 | .02 | .22 |
| Donor type | |||||
| MFD | 0.89 (0.85-0.93) | 0.85 (0.80-0.90) | 0.05 (0.02-0.08) | 0.09 (0.05-0.13) | 0.15 (0.10-0.21) |
| MUD | 0.87 (0.83-0.91) | 0.74 (0.69-0.79) | 0.14 (0.10-0.19) | 0.08 (0.04-0.11) | 0.15 (0.11-0.20) |
| 1-Ag MM | 0.76 (0.67-0.85) | 0.66 (0.55-0.76) | 0.18 (0.09-0.26) | 0.13 (0.06-0.19) | 0.23 (0.14-0.33) |
| >1-Ag MM | 0.79 (0.63-0.95) | 0.62 (0.42-0.82) | 0.24 (0.07-0.42) | 0.03 (0-0.10) | 0.32 (0.05-0.60) |
| P | .02 | <.001 | <.001 | .36 | .24 |
| Stem cell source | |||||
| PB | 0.87 (0.7-0.87) | 0.7 (0.63-0.77) | 0.15 (0.10-0.21) | 0.07 (0.03-0.11) | 0.18 (0.12-0.25) |
| BM | 0.88 (0.85-0.91) | 0.78 (0.74-0.83) | 0.11 (0.08-0.14) | 0.09 (0.06-0.11) | 0.17 (0.13-0.21) |
| CB | 0.82 (0.67-0.96) | 0.62 (0.44-0.8) | 0.27 (0.11 0.44) | 0.14 (0.01-0.27) | 0.17 (0.01-0.33) |
| P | .2 | .006 | .009 | .48 | .92 |
| Year of HCT | |||||
| <2013 | 0.86 (0.83-0.90) | 0.77 (0.73-0.82) | 0.11 (0.08-0.15) | 0.09 (0.06-0.12) | 0.19 (0.14-0.24) |
| ≥2013 | 0.83 (0.79-0.88) | 0.73 (0.67-0.78) | 0.15 (0.10-0.19) | 0.08 (0.05-0.11) | 0.14 (0.10-0.19) |
| P | .4 | .3 | .299 | .63 | .33 |
| . | 3-y OS (95% CI) . | 3-y EFS (95% CI) . | 3-y GF (95% CI) . | Grade III-IV aGVHD (95% CI) . | cGVHD (95% CI) . |
|---|---|---|---|---|---|
| Age | |||||
| <18 y | 0.86 (0.83-0.89) | 0.76 (0.72-0.8) | 0.13 (0.10-0.16) | 0.08 (0.06-0.11) | 0.17 (0.13-0.20) |
| ≥18 y | 0.76 (0.66-0.86) | 0.69 (0.57 0.8) | 0.12 (0.04-0.21) | 0.10 (0.03-0.1) | 0.22 (0.11-0.33) |
| P | .009 | .2 | .74 | 066 | .40 |
| Genetics | |||||
| AR | 0.86 (0.80-0.93) | 0.77 (0.69-0.85) | 0.11 (0.05-0.17) | 0.09 (0.03-0.15) | 0.14 (0.07-0.22) |
| X-linked | 0.85 (0.81-0.89) | 0.75 (0.70-0.80) | 0.14 (0.10-0.18) | 0.08 (0.05-0.11) | 0.15 (0.11-0.20) |
| P | .9 | .7 | .39 | .66 | .65 |
| Colitis | |||||
| Present | 0.78 (0.70-0.86) | 0.72 (0.63-0.81) | 0.09 (0.03-0.14) | 0.11 (0.05-0.17) | 0.18 (0.10-0.26) |
| Absent | 0.87 (0.84-0.91) | 0.76 (0.71-0.8) | 0.15 (0.11-0.19) | 0.08 (0.05-0.11) | 0.14 (0.10-0.18) |
| P | .01 | .5 | .08 | .33 | .31 |
| Liver derangement | |||||
| Present | 0.90 (0.80-0.99) | 0.89 (0.79-0.99) | 0.05 (0-0.13) | 0.05 (0-0.11) | 0.13 (0.01-0.26) |
| Absent | 0.84 (0.81-0.88) | 0.74 (0.69-0.78) | 0.14 (0.10-0.17) | 0.08 (0.06-0.11) | 0.16 (0.12-0.19) |
| P | .4 | .04 | .11 | .38 | .52 |
| Renal derangement | |||||
| Present | 0.76 (0.59-0.92) | 0.75 (0.57-0.92) | 0.16 (0.01-0.31) | 0.08 (0-0.19) | 0.17 (0.01-0.33) |
| Absent | 0.85 (0.81-0.88) | 0.74 (0.7-0.79) | 0.14 (0.10-0.17) | 0.08 (0.05-0.10) | 0.15 (0.11-0.19) |
| P | .1 | .8 | .62 | .99 | .76 |
| Infections | |||||
| Present | 0.83 (0.79-0.88) | 0.74 (0.69-0.79) | 0.13 (0.09-0.17) | 0.08 (0.05-0.12) | 0.14 (0.10-0.18) |
| Absent | 0.87 (0.81-0.92) | 0.74 (0.67-0.81) | 0.14 (0.08-0.20) | 0.06 (0.02-0.10) | 0.16 (0.10-0.23) |
| P | .4 | .9 | .65 | .32 | .62 |
| Conditioning regimen | |||||
| Bu/cy | 0.88 (0.82-0.94) | 0.84 (0.77-0.91) | 0.03 (0.001-0.07) | 0.11 (0.05-0.17) | 0.13 (0.07-0.20) |
| Bu/flu | 0.84 (0.79-0.88) | 0.75 (0.7-0.8) | 0.13 (0.09-0.17) | 0.07 (0.04-0.09) | 0.20 (0.15-0.26) |
| Treo/flu | 0.90 (0.82-0.98) | 0.71 (0.59-0.83) | 0.22 (0.11-0.33) | 0.06 (0.01-0.12) | 0.09 (0.02-0.15) |
| Treo/flu/TT | 0.95 (0.89-1) | 0.85 (0.74-0.95) | 0.10 (0.01-0.19) | 0.09 (0.01-0.16) | 0.07 (0-0.16) |
| P | .09 | .1 | .009 | .38 | .04 |
| In vivo T-cell depletion | |||||
| ATG | 0.91 (0.85-0.97) | 0.84 (0.76-0.93) | 0.09 (0.02-0.16) | 0.05 (0.01-0.10) | 0.18 (0.09-0.27) |
| Alemtuzumab | 0.92 (0.81-1) | 0.88 (0.76-1) | 0.07 (0-0.18) | 0.03 (0-0.09) | 0.04 (0-0.12) |
| None | 0.86 (0.80-0.93) | 0.84 (0.77-0.91) | 0.02 (0-0.06) | 0.15 (0.08-0.22) | 0.16 (0.08-0.24) |
| P | .4 | .8 | .25 | .02 | .22 |
| Donor type | |||||
| MFD | 0.89 (0.85-0.93) | 0.85 (0.80-0.90) | 0.05 (0.02-0.08) | 0.09 (0.05-0.13) | 0.15 (0.10-0.21) |
| MUD | 0.87 (0.83-0.91) | 0.74 (0.69-0.79) | 0.14 (0.10-0.19) | 0.08 (0.04-0.11) | 0.15 (0.11-0.20) |
| 1-Ag MM | 0.76 (0.67-0.85) | 0.66 (0.55-0.76) | 0.18 (0.09-0.26) | 0.13 (0.06-0.19) | 0.23 (0.14-0.33) |
| >1-Ag MM | 0.79 (0.63-0.95) | 0.62 (0.42-0.82) | 0.24 (0.07-0.42) | 0.03 (0-0.10) | 0.32 (0.05-0.60) |
| P | .02 | <.001 | <.001 | .36 | .24 |
| Stem cell source | |||||
| PB | 0.87 (0.7-0.87) | 0.7 (0.63-0.77) | 0.15 (0.10-0.21) | 0.07 (0.03-0.11) | 0.18 (0.12-0.25) |
| BM | 0.88 (0.85-0.91) | 0.78 (0.74-0.83) | 0.11 (0.08-0.14) | 0.09 (0.06-0.11) | 0.17 (0.13-0.21) |
| CB | 0.82 (0.67-0.96) | 0.62 (0.44-0.8) | 0.27 (0.11 0.44) | 0.14 (0.01-0.27) | 0.17 (0.01-0.33) |
| P | .2 | .006 | .009 | .48 | .92 |
| Year of HCT | |||||
| <2013 | 0.86 (0.83-0.90) | 0.77 (0.73-0.82) | 0.11 (0.08-0.15) | 0.09 (0.06-0.12) | 0.19 (0.14-0.24) |
| ≥2013 | 0.83 (0.79-0.88) | 0.73 (0.67-0.78) | 0.15 (0.10-0.19) | 0.08 (0.05-0.11) | 0.14 (0.10-0.19) |
| P | .4 | .3 | .299 | .63 | .33 |
Results are highlighted in bold according to the definition of significance being P ≤ .05.
1-Ag MM, 1-antigen-mismatched donor; BM, bone marrow; Bu, busulfan; CB, cord blood; Cy, cyclophosphamide; Flu, fludarabine; PB, peripheral blood; Treo, treosulfan; TT, thiotepa.
In MVA, OS was lower in older patients (hazard ratio [HR], 1.69; P = .0001, for every 10-year increase in age). Also, OS was significantly reduced in patients who had a 1-antigen-mismatched donor, compared with patients who received a graft from a matched family donor (MFD; HR, 2.29; P = .01) or matched unrelated donor (MUD; HR, 1.8; P = .04; Table 4). There was a trend for reduced OS in patients with colitis before transplantation (HR, 1.72; P = .052).
Multivariate analysis
| . | OS . | EFS . | GF . | grade 3-4 aGVHD . | cGVHD . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| Age (in 10 y) | 1.69 (1.30-2.20) | .0001 | 1.11 (0.87-1.40) | .37 | 0.72 (0.49-1.05) | .09 | 1.25 (0.87-1.80) | .22 | 1.35 (1.05-1.75) | .01 |
| Colitis | ||||||||||
| Absent | 1 | 1 | 1 | 1 | 1 | |||||
| Present | 1.72 (0.99-2.99) | .052 | 1.03 (0.65-1.63) | .87 | 0.50 (0.23-1.08) | .08 | 1.38 (0.66-2.84) | .38 | 1.24 (0.70-2.21) | .45 |
| Conditioning regimen | ||||||||||
| Bu/Flu | 1 | 1 | 1 | 1 | 1 | |||||
| Bu/Cy | 1.22 (0.62-2.39) | .54 | 0.81 (0.46-1.42) | .48 | 0.31 (0.11-0.91) | .03 | 2.13 (1.03-4.39) | .04 | 0.81 (0.43-1.52) | .52 |
| Treo/Flu | 0.83 (0.40-1.71) | .62 | 1.11 (0.68-1.81) | .67 | 1.38 (0.7459-2.56) | .30 | 1.82 (0.85-3.89) | .12 | 0.73 (0.36-1.45) | .37 |
| Treo/Flu/TT | 0.32 (0.07-1.34) | .11 | 0.62 (0.28-1.36) | .23 | 0.78 (0.30-2.03) | .62 | 1.56 (0.58-4.16) | .37 | 0.35 (0.11-1.15) | .08 |
| Other | 1.04 (0.48-2.27) | .90 | 1.62 (0.93-2.80) | .08 | 2.00 (1.001-4.01) | .04 | 1.2759 (0.43-3.77) | .65 | 1.33 (0.63-2.79) | .44 |
| Donor type | ||||||||||
| MFD | 1 | 1 | 1 | 1 | 1 | |||||
| MUD | 1.27 (0.71-2.25) | .40 | 1.89 (1.19-2.98) | .006 | 2.33 (1.19-4.57) | .01 | 1.07 (0.57-2.00) | .82 | 1.02 (0.62-1.69) | .91 |
| 1-Ag mismatch | 2.29 (1.18-4.42) | .01 | 2.37 (1.38-4.08) | .001 | 2.67 (1.22-5.87) | .01 | 1.67 (0.77-3.61) | .1 | 1.82 (0.98-3.38) | .057 |
| >1-Ag mismatch | 2.46 (0.78-7.72) | .12 | 3.69 (1.65-8.22) | .001 | 5.54 (1.92-15.99) | .001 | 0.57 (0.07-4.52) | .59 | 1.94 (0.63-5.95) | .24 |
| Stem cell source | ||||||||||
| BM | 1 | 1 | 1 | 1 | 1 | |||||
| CB | 1.05 (0.36-3.06) | .92 | 1.22 (0.57-2.61) | .60 | 1.27 (0.48-3.33) | .62 | 1.97 (0.66-5.86) | .22 | 0.61 (0.18-2.03) | .42 |
| PB | 1.02 (0.59-1.76) | .92 | 1.14 (0.76-1.71) | .51 | 1.08 (0.62-1.88) | .77 | 0.75 (0.37-1.51) | .42 | 0.82 (0.48-1.42) | .49 |
| . | OS . | EFS . | GF . | grade 3-4 aGVHD . | cGVHD . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| Age (in 10 y) | 1.69 (1.30-2.20) | .0001 | 1.11 (0.87-1.40) | .37 | 0.72 (0.49-1.05) | .09 | 1.25 (0.87-1.80) | .22 | 1.35 (1.05-1.75) | .01 |
| Colitis | ||||||||||
| Absent | 1 | 1 | 1 | 1 | 1 | |||||
| Present | 1.72 (0.99-2.99) | .052 | 1.03 (0.65-1.63) | .87 | 0.50 (0.23-1.08) | .08 | 1.38 (0.66-2.84) | .38 | 1.24 (0.70-2.21) | .45 |
| Conditioning regimen | ||||||||||
| Bu/Flu | 1 | 1 | 1 | 1 | 1 | |||||
| Bu/Cy | 1.22 (0.62-2.39) | .54 | 0.81 (0.46-1.42) | .48 | 0.31 (0.11-0.91) | .03 | 2.13 (1.03-4.39) | .04 | 0.81 (0.43-1.52) | .52 |
| Treo/Flu | 0.83 (0.40-1.71) | .62 | 1.11 (0.68-1.81) | .67 | 1.38 (0.7459-2.56) | .30 | 1.82 (0.85-3.89) | .12 | 0.73 (0.36-1.45) | .37 |
| Treo/Flu/TT | 0.32 (0.07-1.34) | .11 | 0.62 (0.28-1.36) | .23 | 0.78 (0.30-2.03) | .62 | 1.56 (0.58-4.16) | .37 | 0.35 (0.11-1.15) | .08 |
| Other | 1.04 (0.48-2.27) | .90 | 1.62 (0.93-2.80) | .08 | 2.00 (1.001-4.01) | .04 | 1.2759 (0.43-3.77) | .65 | 1.33 (0.63-2.79) | .44 |
| Donor type | ||||||||||
| MFD | 1 | 1 | 1 | 1 | 1 | |||||
| MUD | 1.27 (0.71-2.25) | .40 | 1.89 (1.19-2.98) | .006 | 2.33 (1.19-4.57) | .01 | 1.07 (0.57-2.00) | .82 | 1.02 (0.62-1.69) | .91 |
| 1-Ag mismatch | 2.29 (1.18-4.42) | .01 | 2.37 (1.38-4.08) | .001 | 2.67 (1.22-5.87) | .01 | 1.67 (0.77-3.61) | .1 | 1.82 (0.98-3.38) | .057 |
| >1-Ag mismatch | 2.46 (0.78-7.72) | .12 | 3.69 (1.65-8.22) | .001 | 5.54 (1.92-15.99) | .001 | 0.57 (0.07-4.52) | .59 | 1.94 (0.63-5.95) | .24 |
| Stem cell source | ||||||||||
| BM | 1 | 1 | 1 | 1 | 1 | |||||
| CB | 1.05 (0.36-3.06) | .92 | 1.22 (0.57-2.61) | .60 | 1.27 (0.48-3.33) | .62 | 1.97 (0.66-5.86) | .22 | 0.61 (0.18-2.03) | .42 |
| PB | 1.02 (0.59-1.76) | .92 | 1.14 (0.76-1.71) | .51 | 1.08 (0.62-1.88) | .77 | 0.75 (0.37-1.51) | .42 | 0.82 (0.48-1.42) | .49 |
HR and 95% CI are from Cox regression analysis. Results are highlighted in bold according to the definition of significance being P < .05.
1-Ag MM, 1-antigen-mismatched donor; BM, bone marrow; Bu, busulfan; CB, cord blood; Cy, cyclophosphamide; Flu, fludarabine; PB, peripheral blood; Treo, treosulfan; TT, thiotepa.
Patients with colitis died of infections (44%), infections and GVHD (9%), GVHD (26%), or toxicity (21%).
Donor engraftment and event-free survival
Donor engraftment was achieved in 610 of 694 (88%) evaluable patients, whereas 84 of 694 (12%) patients experienced primary or secondary GF. The median time for neutrophil and platelet recovery was 18 days (range, 3-133) and 19 days (range, 3-131), respectively (Table 2). The EFS at 3 years was 75.8% (95% CI, 72.3-79.3; Figure 1B). Supplemental Figure 1 (available on the Blood Web site) shows EFS including chronic extensive GVHD as an event.
In UVA, EFS was influenced by donor type (P < .001), stem cell source (P = .006), and liver derangement pre-HCT (P = .04; Table 3; Figure 1D,F).
In MVA, EFS correlated significantly with donor type, when compared with the use of a graft from an MFD. The use of a graft from an MUD (HR, 1.89; P = .006), 1-antigen-mismatched donor (HR, 2.37; P = .001), and >1-antigen-mismatched donor (HR, 3.69; P = .001) all with significantly decreased EFS (Table 4).
In UVA, GF was influenced by donor type (P < .001), choice of conditioning regimen (P = .009), and stem cell source (P = .009; Table 3).
In MVA, GF correlated significantly with donor type, when compared to the use of a graft from an MFD. The use of a graft from an MUD (HR, 2.33; P = .01), a 1-antigen-mismatched donor (HR, 2.67; P = .01), and a >1 antigen-mismatched donor (HR, 5.54; P = .001) all significantly increased the risk of GF (Table 4). Also, GF was significantly reduced in patients who received busulfan and cyclophosphamide, when compared to those who received busulfan and fludarabine (HR, 0.31; P = .03; Table 4).
GVHD
The cumulative incidence of grades II-IV and grades III-IV aGVHD was 20.1% (95% CI, 17.1-23.2) and 9% (95% CI, 6.9-11.2), respectively (Figure 1G). The cumulative incidence of cGVHD and extensive cGVHD at 3 years was 17.8% (95% CI, 14.5-21.2) and 6.2% (95% CI, 4.1-8.3), respectively (Figure 1H).
In MVA, grade III-IV aGVHD was significantly higher in patients who received busulfan and cyclophosphamide, compared with those who received busulfan and fludarabine (HR, 2.13; P = .04). Also, cGVHD was significantly higher in older patients (HR, 1.35; P = .01; Table 4). There was a trend toward increased risk of cGVHD in patients who received grafts from 1-antigen-mismatched donors, compared with those who received transplants from MFDs (HR, 1.82; P = .057).
Second transplantation
Ninety-eight patients underwent a second allo-HCT because of graft rejection or progressive falling chimerism: the 3-year OS and EFS were 76.6% (95% CI, 67-86.3) and 75.9% (95% CI 65.1-86.7), respectively, after the second HCT (data not shown).
Discussion
Patients with CGD have chronic, invasive, opportunistic infections and severe autoinflammatory complications, leading to poor quality of life and reduced survival time, despite adequate supportive care.13,14 Allo-HCT can cure CGD, with resolution of infections and immune dysregulation,1 but indication for and timing of transplantation have been controversial over the years, because of the chronic course of the disease with conventional treatment and relatively scarce data on transplantation. Given that the last EBMT retrospective study on allo-HCT in CGD, published in 2002, described only 27 transplant recipients, it is apparent that the frequency of transplantations has increased over the past 2 decades,15 most likely because of the improved outcome after HCT.6,8,9,16-18
We analyzed the outcomes of 712 children and adults with CGD who underwent allo-HCT and defined significant risk factors. Interestingly, 87% of the patients in this study received a transplant after 2006, indicating a recent and significant change of practice in the management of patients with CGD.
Overall, our data show excellent survival and an acceptable incidence of acute and chronic GVHD.
Our study included an exceptionally large number of adult patients (n = 77). The OS and EFS at 3 years for patients ≥18 years was 76% (95% CI, 66-86) and 69% (95% CI, 57-80), respectively. Although we demonstrated that transplant-related mortality and the risk of cGVHD increased with age and that survival was significantly higher in children, adults are still predicted to benefit from transplantation, and this treatment option should be strongly considered. However, our findings demonstrate that, preferably, transplantation should be discussed early with patients and/or their families to maximize a successful outcome.
Previous reports have shown a milder clinical course for patients with AR CGD, leading to longer survival, when compared with X-linked CGD.17,18 In our study, that the pattern of genetic inheritance did not influence outcome after transplantation.
Most patients in our study had CGD-related comorbidities before receiving the transplant, including infections, colitis, and liver or renal derangement. Because of the retrospective nature of this study a proportion of the data were missing, therefore limiting its interpretation. In particular, no formal performance scores before transplantation were available; those data may be useful in the future. Patients with chronic inflammatory bowel disease had a trend toward reduced survival compared with those without colitis (HR, 1.72; P = .052), consistent with previous reports describing poor outcome in patients with significant pre-HCT inflammation.15,19 This is in contrast with a recent study by Marsh et al20 who compared 49 patients who had CGD with inflammatory bowel disease with 96 patients without, all of whom underwent allo-HCT. No significant difference was found in engraftment, upper or lower acute GVHD, and cGVHD between the 2 groups, and 5-year OS was equivalent. Patients with granulomatous colitis may benefit from an optimization of their immunosuppressive strategy before transplantation, to reduce the inflammatory burden.21 This notion should be carefully balanced against the individualized risk of opportunistic infections. As in Marsh et al,20 the presence of colitis in our cohort did not translate into an increased risk of acute or chronic GVHD, possibly because of the extensive use of in vivo T-cell depletion (ATG or alemtuzumab) in recent transplantation protocols. Information on resolution of pretransplantation colitis after the transplant was performed was not documented for our study, but has been reported.8,19
One of the main end points of this study was to assess the impact of different conditioning regimens on transplantation outcomes. Whereas earlier reports on allo-HCT in CGD described the use of myeloablative preparative regimens,15,19 in recent years, reduced toxicity protocols with either busulfan8 or treosulfan9 have been adopted with good outcome in donor engraftment and reduction of transplant-related mortality. We analyzed the influence of the 4 most commonly used conditioning regimens on transplantation outcomes (Table 4). There was no significant impact on OS and EFS, although the use of myeloablative busulfan and cyclophosphamide was associated with a reduced risk of GF, compared with the use of busulfan and fludarabine. Data on busulfan pharmacokinetics were collected in only a small proportion of patients, and therefore a correlation between busulfan systemic exposure and GF was not feasible. Moreover, the combination of busulfan and cyclophosphamide was associated with an increased risk of severe aGVHD compared with busulfan and fludarabine, most likely because of the reduced use of in vivo T-cell depletion within this protocol. With no significant impact of the choice of conditioning regimen on OS and EFS and given the short- and long-term toxicity (infertility and secondary malignancies) expected with a myeloablative conditioning regimen,22,23 a reduced toxicity busulfan or treosulfan-based approach should be preferred in patients with CGD. Details of serotherapy, used together with timing and doses plus myeloid chimerism and immune reconstitution data, were not available. Those data would be important in refining the conditioning regimens, to minimize short-term and late effects, optimize engraftment and immune reconstitution, and minimize GVHD.24,25
One of the strengths of this study was the enrollment of patients who received transplants from different donor types, with a significant number of HLA-mismatched transplants (n = 134). For the first time, the availability of this information allowed for a detailed analysis of the impact of donor type and degree of HLA mismatch on different transplant outcomes. These data are of paramount importance, when counseling patients with CGD and their families on allo-HCT or gene therapy.
In our study, patients undergoing allo-HCT from an MFD had the best outcome, with a 3-year OS of 89.4% and an EFS of 85.3%, consistent with previous reports of smaller samples.8,9 Patients receiving a transplant from a 10/10 HLA-MUD had similar 3-year OS (87.7%), but reduced 3-year EFS (74.4%), compared with those with an MFD (HR, 1.89; P = .006), because of an increased risk of GF.
Importantly, patients receiving transplants from 1-antigen-mismatched donors (n = 105) had reduced survival compared with MFDs (HR, 2.29; P = .01) or MUDs (HR, 1.8; P = .04). This was not observed in patients who received transplants from donors with a >1 antigen mismatch, probably because of the small number of patients in this group (n = 29). Notably, the group of patients receiving transplants from 1-antigen-mismatched donors had late deaths (>2 years after transplantation; Figure 1E), mostly related to GVHD. As 89% of grafts from 1-antigen-mismatched donors were not manipulated (no in vitro T-cell depletion; Table 1), it is possible that a more profound in vivo and in vitro T-cell depletion would protect patients who receive highly mismatched transplants against GVHD-related death. Therefore, as transplant-related mortality is higher with the use of 1-antigen-mismatched donors, indication for transplantation in this setting should be carefully assessed by the treating physicians, and deeper T-cell depletion strategies should be considered.
Patients who received a graft from donors with >1 antigen mismatch had a significantly reduced 3-year EFS (HR, 3.69; P = .001) and increased risk of GF (HR, 5.54; P = .001) compared with those who had an MFD. Nevertheless, this group was small and very heterogeneous in terms of degree of HLA mismatch, as well as adopted strategies for T-cell depletion. Given the promising outcomes reported with novel approaches for graft manipulation in the context of haploidentical hematopoietic cell transplantation,26-29 it is likely that results in this setting will improve over time in experienced centers. An alternative is to consider gene therapy or gene editing, the results of which are emerging and will become more widely available with time.30,31
In summary, this study is the largest analysis to date on outcome of children and adults with CGD after allo-HCT and represents a guidance for clinicians in counseling patients and their families. We demonstrated an excellent outcome after allo-HCT in CGD, with a low incidence of GF and mortality for all ages. However, older patients and recipients of HLA-mismatched grafts showed a less favorable outcome; therefore, transplantation should be strongly considered and discussed at a younger age, particularly in the presence of a well-matched donor. After balancing individualized risks and benefits, treating physicians should very carefully assess the use of HLA-mismatched donors and modify their protocols accordingly.
Original data are available by e-mail request to the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank all the contributing centers listed in the supplemental file.
Authorship
Contribution: R.C., T.G., and M.A.S. conceived and designed the study; H.-J.B. and S.H. managed the data; J.W. performed the statistical analysis; R.C., T.G., M.A.S., P.V., A.R.G., M.H.A., A.L., J.W., and S.H. analyzed the data and wrote the manuscript; and R.C., T.G., M.A.S., P.V., A.R.G., M.H.A., A.L., B.N., D.M., A.S., M.H., F.H., A.A.S., J.G., P.L., C.A.L., J.F.F., K.K., B.S., U.S., P. Sedlacek, K.-W.S., S.A., F.L., P. Stepensky, R.W., S.H.L., M.Z., F.P., M.T., B.G., S.M., M.K., M.H.-H., G.L., D.B., and M.F. contributed clinical data and reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of the leadership of the Inborn Errors Working Party of the EBMT appears in the supplemental appendix.
Correspondence: Mary A. Slatter, Paediatric Immunology, Infectious Diseases and Allergy Department, Clinical Resource Building, Block 2, Level 4, Royal Victoria Infirmary, Queen Victoria Rd, Newcastle upon Tyne NE1 4LP, United Kingdom; e-mail: mary.slatter@nhs.net.
REFERENCES
Author notes
T.G. and M.A.S. contributed equally to this study.