Abstract
Gastrointestinal (GI) bleeding is distinctive of severe von Willebrand disease (VWD), generally arising in older patients; in most cases, blood transfusion and hospitalization are required. The presence of arteriovenous malformations is often described when endoscopic examinations are performed. Patients with congenital type 3, 2A, and 2B are those most frequently affected by this symptom, possibly due to the loss of high-molecular-weight multimers of von Willebrand factor (VWF). GI bleeding can also occur in patients affected by acquired von Willebrand syndrome. Endoscopic examination of the GI tract is necessary to exclude ulcers and polyps or cancer as possible causes of GI bleeding. In congenital VWD, prophylaxis with VWF/factor VIII concentrates is generally started after GI-bleeding events, but this therapy is not always successful. Iron supplementation must be prescribed to avoid chronic iron deficiency. Possible rescue therapies (high-dose statins, octreotide, thalidomide, lenalidomide, and tamoxifen) were described in a few case reports and series; however, surgery may be necessary in emergency situations or if medical treatment fails to stop bleeding. In this article, we present several clinical cases that highlight the clinical challenges of these patients and possible strategies for their long-term management.
Introduction
von Willebrand disease (VWD) is caused by the congenital deficiency of von Willebrand factor (VWF), a multimeric protein involved in platelet adhesion and blood coagulation (by transporting factor VIII [FVIII] and preserving it from clearance).1 VWD is classified into type 1 (partial quantitative deficiency with normal multimer profile), type 2 (qualitative deficiency, characterized by a discrepancy between function and antigen), and type 3 (severe quantitative deficiency).2 At present, in cases with severe VWD, patients are treated with plasma-derived concentrates containing FVIII and VWF (or VWF only) and the recently introduced recombinant VWF concentrate (Vonvendi). Adjuvant therapies such as tranexamic acid are also used, especially in cases of mucosal bleeding.3,4
Acquired von Willebrand syndrome (AVWS) is a rare bleeding disorder, associated with several underlying diseases and different pathogenic mechanisms. In patients with hypothyroidism, AVWS is associated with decreased VWF synthesis, whereas in autoimmune diseases and in monoclonal gammopathies, VWF is normally produced but its clearance is increased by antibodies. In cases with aortic stenosis or left-ventricular assist devices (LVADs), increased VWF clearance is a result of high shear stress in the heart or a device that unfolds VWF, increasing its susceptibility to proteolysis by ADAMTS-13.5,6
Recurrent gastrointestinal (GI) bleeding is a distinctive symptom of VWD, especially in the elderly. The VWD Prophylaxis Network (PN) study reported angiodysplasia in 38% of the patients with at least 1 episode of GI bleeding7 ; however, a specific diagnosis may be challenging, requiring multiple endoscopic procedures. A large survey of 4503 VWD patients published in 1993 evaluated the association between VWD type and angiodysplasia, reporting angiodysplasia in 0% of type 1 patients, 2% of type 2 patients, 4.5% of type 3 VWD patients, and in 11.5% of those with the AVWS.8 This finding suggests the importance of an intact VWF multimeric structure to avoid GI bleeding. This hypothesis is supported by Castaman et al,9 who compared 46 type 2A patients (who lack high-molecular-weight multimers [HMWMs]) and 61 type 2M patients (who have normal multimers): in a period of 24 months, 37% of type 2A patients had GI bleeding, compared with 3% of type 2M patients. The role of VWF in the regulation of angiogenesis was described,10 showing the molecular and cellular pathways whereby VWF regulates endothelial cell proliferation, migration, and sprouting. Whether these mechanisms are more influenced by the amount of VWF that is available or by its quality (presence or absence of HMWMs or presence of a defective protein that causes defective angiogenesis) remains to be understood.
Diagnosis, treatment, and prophylaxis with VWF/FVIII concentrate
Case 1
A 48-year-old woman with type 3 VWD was admitted for acute GI bleeding and anemia (melena and hemoglobin, 7 g/dL). The endoscopic study of her upper and lower GI tract found no ulcer nor active bleeding susceptible to endoscopic treatment. Double-balloon enteroscopy was performed and gave evidence of prepyloric and duodenal bleeding in the absence of ulcers (with no clear evidence of angiodysplasia).
Recent medical history
In the previous 3 years, 2 episodes of melena required red blood cell transfusions (4 U). Between events, the patient had mild to moderate chronic anemia, caused by iron deficiency. Cycles of oral iron therapy were recommended, with good correction of ferritin and hemoglobin levels. The patient denied evident traces of blood in her stools and refused to collect stools for occult blood testing.
Comorbidities
The patient received follow-up care for hepatitis C virus infection (caused by previous transfusions) and systemic lupus erythematosus. She presented with important arthropathy in her ankles and elbows caused by repeated joint bleeds since youth.
Therapy
During the acute phase of bleeding, she received 6 U of red blood cells, supplementation with iron and folic acid, and hemostatic treatment with a VWF/FVIII concentrate (Humate-P), maintaining FVIII coagulant (FVIII:C) >50 IU/dL and VWF ristocetin cofactor (VWF:RCo) >30 IU/dL in the first 7 days. A prophylactic regimen was proposed, but she refused and was discharged with oral supplementation of iron and folic acid.
Follow-up
The patient presented with recurrent GI bleeding, requiring hospitalization, red blood cell transfusion, and treatment with VWF/FVIII concentrates (4 episodes in 6 months with a total of 26 U of red blood cells transfused). Upper GI tract endoscopy was performed at admission, finding an angiodysplastic lesion of the duodenum that was treated with argon-plasma coagulation. On subsequent admission, duodenal bleeding without evident lesions (sine materia) was found at endoscopy and treated with hemoclips.
The patient finally accepted a prophylactic regimen with VWF/FVIII concentrate (50 IU of FVIII per kilogram 3 times per week). In the following 12 months, she had 1 episode of acute GI bleeding, requiring 4 U of red blood cells, and needed cycles of iron therapy for the following 2 years. The prophylactic regimen with Humate-P was reduced to 50 IU of FVIII per kilogram twice per week after 24 months (and to 40 IU of FVIII per kilogram twice per week after an 2 additional years). The patient had no other episode of acute GI bleeding in the next 5 years and received no more iron therapy.
Discussion
Prophylaxis with a VWF/FVIII concentrate was effective in this patient. Nevertheless, other studies11-13 showed that prophylaxis is less efficacious for GI bleeding compared with hemarthrosis (49% vs 86%).11 If we hypothesize that VWF deficiency causes an increased angiogenesis that leads to angiodysplasia, we would also expect that the vascular remodeling initiated by the prophylactic regimen would take some time. For this reason, the effectiveness of prophylaxis should be evaluated after a minimum of 12 months. Furthermore, we know that all plasma-derived VWF/FVIII concentrates that were used in the few published studies lack HMWMs, and this could be another reason for their poor efficacy in patients with GI bleeding, especially in the short-term. Accordingly, the new recombinant VWF concentrate (Vonvendi), which contains all multimers (and also ultra-large multimers), could be more effective in controlling GI bleeding, but this hypothesis has not yet been validated.
No evidence-based recommendation can be issued at the moment on the best timing to start (and also to stop) a prophylactic regimen, nor on its dosing. It is our practice to initiate a prophylactic regimen (30-50 IU of VWF per kilogram 2-3 times per week) after 2 episodes of GI bleeding (acute or chronic bleeding) and to carry it on for at least 2 years. The presence of other bleeding symptoms or arthropathy is reason enough to carry it on even longer. In cases of severe type 3 VWD characterized by low levels of FVIII:C (<5 IU/dL) and repeated joint bleeding, it is our current policy to begin prophylaxis in young patients. In the future, evaluating whether this early strategy will be able to prevent GI bleeding later in life will be interesting.
Rescue therapy in association with VWF/FVIII concentrate in congenital VWD
Case 2
An 83-year-old woman with type 2B VWD (FVIII:C, 57%; VWF antigen [VWF:Ag], 52%; VWF:RCo, 10%; and loss of HMWMs, gene mutation p.R1306W, and transient thrombocytopenia) was admitted for acute anemia and melena (hemoglobin, 7 g/dL). The endoscopic study of the upper GI tract found gastritis and several foci of gastric bleeding that were partially treated with argon-plasma coagulation and hemoclips.
Recent medical history
In the previous 4 years, the patient had experienced 4 episodes of anemia associated with melena (on 1 occasion) or stools positive for blood. She was hospitalized and transfused with red blood cells on 1 occasion (2 U); because of an upper GI endoscopy positive for acute gastritis, she was treated with a proton-pump inhibitor.
Comorbidities
The patient’s comorbidities included chronic atrial fibrillation and hepatitis C virus–related liver cirrhosis.
Therapy
The patient received red blood cell transfusion (3 U), IV iron supplementation, folic acid, VWF/FVIII concentrate (Humate-P, maintaining in the first 7 days VWF:RCo >30 IU/dL and avoiding FVIII:C >150 IU/dL), oral esomeprazole, and tranexamic acid. After discharge, she received esomeprazole and folic acid in association with IV iron supplementation.
Follow-up
The patient had recurrent GI bleeding with the need for red blood cell transfusions and episodic treatment with VWF/FVIII concentrates (3 episodes in 3 months, 10 U of red blood cells transfused). She underwent upper GI tract endoscopy that led to the treatment of bleeding (clips and argon-plasma coagulation for diffuse bleeding on the first occasion, and subsequent treatment of lesions of the greater and lesser curvature with argon-plasma coagulation and hemoclips).
After 3 months, because of the frequent bleeding episodes, she was started on prophylaxis with a plasma-derived VWF/FVIII concentrate (Humate-P, 40 IU of FVIII per kilogram 3 times per week). Despite prophylaxis, she had more episodes of recurrent acute GI bleeding, the need for red blood transfusion, and hospitalization (4 occasions in the following 5 months, 20 U of red blood cells transfused). Because of the high transfusional need and poor quality of life, she was started on 80mg of high-dose atorvastatin per day. This therapy was continued for 6 months and then stopped for lack of efficacy (5 episodes of acute GI bleeding in 6 months, 20 U of red blood cells transfused). It was then decided that the patient would start 5 mg of lenalidomide per day, per 3 weeks, every 4 weeks, despite the thrombotic risk associated with atrial fibrillation. The patient had a prompt reduction in GI bleeding in the next 4 months (only 2 U of red blood cells transfused soon after the beginning of this therapy) but she developed neutropenia (<500/mm3) after 5 months. After lenalidomide was stopped, GI bleeding restarted, with the associated need for weekly red blood transfusion every 7 to 14 days (follow-up, 8 months).
Discussion
Control of GI bleeding can be particularly difficult in some patients: this was also reported in the PRO.WILL study, in which a single patient (in a group of 10) had 9 GI-bleeding episodes (64% of the events) despite a prophylactic regimen.13 In cases of recurrent acute GI bleeding that may be life-threatening and cause a significant worsening in quality of life, the clinician might choose a rescue therapy. Unfortunately, this choice can only rely on case reports or small case series, with the unavoidable publication bias of unsuccessful cases. In making the choice, the clinician must consider the whole medical history of the patient as well as comorbidities and the possible side effects of the rescue therapy. Table 1 reports several rescue treatments used in patients with VWD and recurrent GI bleeding. Another important issue is when to stop prophylaxis and/or the rescue treatment. Unfortunately, no data are available on the long-term follow-up of these challenging patients. In patients with type 2 VWD, the decision to carry on the prophylactic regimen may be difficult. Our opinion and practice in these patients, in the absence of evidence-based data, is to carry it on for at least 1 year since the last bleeding episode, to perform the search for occult blood in the stools, and to evaluate the full blood count and iron balance every 6 months after stopping prophylaxis, so that possible chronic GI bleeding can be diagnosed and handled early.
Rescue therapies in congenital VWD and AVWS associated with monoclonal gammopathy or autoimmune diseases
| Therapy patient no. . | Dose . | Outcome and follow-up . | Previous unsuccessful therapies . | Possiblemechanism . | Reference . |
|---|---|---|---|---|---|
| Estrogen/ progesterone | |||||
| 1 (type 2B VWD) | Estradiol patch (50 µg twice/wk for 3 wk on and 1 wk off), medroxyprogesterone acetate 5 mg/d (days 16-21) | Control of GI bleeding; follow-up, 11 mo | Not reported | VWF and FVIII increase | 30 |
| Danazol | |||||
| 2 (type 2B VWD) | 500 mg/d | Pt 1: control of GI bleeding but discontinued after 2 y for severe liver toxicity; follow-up, 6 y | Pt 1: VWF/FVIII concentrate prophylaxis, ε-aminocaproic acid, octreotide | FVIII increase | 31 |
| Pt 2: control of GI bleeding; follow-up, 18 mo | Pt 2: VWF/FVIII concentrate prophylaxis | ||||
| Tamoxifen | |||||
| 2 (type 3 VWD) | Not reported (possibly 20 mg/d) | Pt 1: control of GI bleeding; follow-up, 14 mo | Pt 1: VWF/FVIII concentrate prophylaxis, thalidomide, propranolol and isosorbide mononitrate, atorvastatin | Antiangiogenic effect | 32 |
| Pt 2: control of GI bleeding; follow-up, 10 mo (therapy stopped after 4 mo) | Pt 2: VWF/FVIII concentrate prophylaxis, statins | ||||
| Recombinant activated FVII | |||||
| 1 (type 1) | 80 µg/kg once daily during acute bleeding, associated with VWF/FVIII concentrate prophylaxis | Control of GI bleeding; follow-up, 2 y | Estrogen, desmopressin, tranexamic acid, VWF/FVIII concentrate prophylaxis, surgery | Activation of alternative hemostatic pathway | 33 |
| High-dose atorvastatin | |||||
| 1 (severe type 1 VWF) | 40 mg/d | Control of GI bleeding; follow-up, 12 mo | VWF/FVIII concentrate prophylaxis, thalidomide 100 mg/d discontinued for side effects, octreotide 20 mg/mo discontinued for lack of efficacy | Antiangiogenic effect at high dose | 34 |
| 1 (type 2A VWD) | 80 mg/d | Control of GI bleeding; follow-up, 14 mo | Ethinylestradiol, thalidomide | Antiangiogenic effect at high dose | 35 |
| Octreotide | |||||
| 2 (type 1, type 2A) | IV 500 µg for 2 d, 250 µg/thrice daily (subcutaneous), then tapered to lower dose | Pt 1: control of GI bleeding; follow-up, 13 mo | Pt 1: VWF/FVIII concentrate prophylaxis, tranexamic acid, desmopressin, high-dose estrogens | Reduction of splanchnic and portal blood flow | 36 |
| Pt 2: control of GI bleeding; follow-up, 6 mo | Pt 2: VWF/FVIII concentrate | ||||
| 1 (type 1 VWD) | 20 mg intramuscular every month | Control of GI bleeding (in association with propranolol 20 mg/3 times per day); follow-up, 8 mo | Desmopressin prophylaxis | Reduction of splanchnic and portal blood flow | 37 |
| Thalidomide | |||||
| 1 (type 2B VWD) | 150 mg/d | Control of GI bleeding; follow-up, 5 mo | Octreotide, discontinued for diarrhea | Antiangiogenic effect | 38 |
| 1 (type 2A) | 100 mg/d, increased to 150 mg/d for bleeding | Initial control of GI bleeding for 1 y, subsequent recurrence; follow-up, 22 mo | Desmopressin, tranexamic acid, VWF/FVIII concentrate | Antiangiogenic effect | 39 |
| 1 (type 2B) | 100 mg/d, decreased to 50 mg/d for fatigue | Control of GI bleeding (in association with estradiol/norethisterone, tranexamic acid), follow-up | VWF concentrate prophylaxis, recombinant activated FVII | Antiangiogenic effect | 40 |
| 1 (AVWS associated to IgG monoclonal gammopathy) | 50 mg/d | Control of GI bleeding; follow-up, 3 y | Desmopressin, VWF/FVIII concentrate, IV immunoglobulins, tranexamic acid, propranolol | Antiangiogenic effect | 41 |
| Lenalidomide | |||||
| 5 (3 type 3 VWD, 1 type 1 VWD, 1 type 2A VWD) | 5 mg/d for 3 wk on and 1 wk off, uptitrated to 10 and 15 mg/d if necessary (tapering to 2 wk on and 2 off when GI bleeding was obtained) | Control of GI bleeding; follow-up, 4-24 mo | Not reported | Antiangiogenic effect | 42 |
| 1 (AVWS associated to IgM monoclonal gammopathy) | 25 mg/d for 3 wk on and 1 wk off (decreased to 20 mg/d for 3 wk on and 1 wk off) | Control of GI bleeding; follow-up, 11 mo | Tranexamic acid, VWF/FVIII concentrate prophylaxis, plasma exchange, IV immunoglobulins, atorvastatin, octreotide, rituximab, and bendamustine | Antiangiogenic effect | 43 |
| Rituximab | |||||
| 2 (AVWS associated to monoclonal gammopathy) | Pt 1: 350 mg/m2 for 2 administrations | Not efficacious | Successful treatment with IV immunoglobulins | Anti-CD20, immunosuppressive effect | 44 |
| Pt 2: 350 mg/m2 for 4 administrations | |||||
| 1 (AVWS associated to systemic lupus erythematosus) | 375 mg/m2 biweekly in 2 doses | Control of GI bleeding and normalization of VWF levels | Steroid and cyclophosphamide | Anti-CD20, immunosuppressive effect | 45 |
| 1 (AVWS, characteristics not specified) | 375 mg/m2 weekly for 4 wk | Control of GI bleeding; follow-up, 5 mo | VWF concentrate, thalidomide; successful treatment with IV immunoglobulins | Anti-CD20, immunosuppressive effect | 46 |
| Therapy patient no. . | Dose . | Outcome and follow-up . | Previous unsuccessful therapies . | Possiblemechanism . | Reference . |
|---|---|---|---|---|---|
| Estrogen/ progesterone | |||||
| 1 (type 2B VWD) | Estradiol patch (50 µg twice/wk for 3 wk on and 1 wk off), medroxyprogesterone acetate 5 mg/d (days 16-21) | Control of GI bleeding; follow-up, 11 mo | Not reported | VWF and FVIII increase | 30 |
| Danazol | |||||
| 2 (type 2B VWD) | 500 mg/d | Pt 1: control of GI bleeding but discontinued after 2 y for severe liver toxicity; follow-up, 6 y | Pt 1: VWF/FVIII concentrate prophylaxis, ε-aminocaproic acid, octreotide | FVIII increase | 31 |
| Pt 2: control of GI bleeding; follow-up, 18 mo | Pt 2: VWF/FVIII concentrate prophylaxis | ||||
| Tamoxifen | |||||
| 2 (type 3 VWD) | Not reported (possibly 20 mg/d) | Pt 1: control of GI bleeding; follow-up, 14 mo | Pt 1: VWF/FVIII concentrate prophylaxis, thalidomide, propranolol and isosorbide mononitrate, atorvastatin | Antiangiogenic effect | 32 |
| Pt 2: control of GI bleeding; follow-up, 10 mo (therapy stopped after 4 mo) | Pt 2: VWF/FVIII concentrate prophylaxis, statins | ||||
| Recombinant activated FVII | |||||
| 1 (type 1) | 80 µg/kg once daily during acute bleeding, associated with VWF/FVIII concentrate prophylaxis | Control of GI bleeding; follow-up, 2 y | Estrogen, desmopressin, tranexamic acid, VWF/FVIII concentrate prophylaxis, surgery | Activation of alternative hemostatic pathway | 33 |
| High-dose atorvastatin | |||||
| 1 (severe type 1 VWF) | 40 mg/d | Control of GI bleeding; follow-up, 12 mo | VWF/FVIII concentrate prophylaxis, thalidomide 100 mg/d discontinued for side effects, octreotide 20 mg/mo discontinued for lack of efficacy | Antiangiogenic effect at high dose | 34 |
| 1 (type 2A VWD) | 80 mg/d | Control of GI bleeding; follow-up, 14 mo | Ethinylestradiol, thalidomide | Antiangiogenic effect at high dose | 35 |
| Octreotide | |||||
| 2 (type 1, type 2A) | IV 500 µg for 2 d, 250 µg/thrice daily (subcutaneous), then tapered to lower dose | Pt 1: control of GI bleeding; follow-up, 13 mo | Pt 1: VWF/FVIII concentrate prophylaxis, tranexamic acid, desmopressin, high-dose estrogens | Reduction of splanchnic and portal blood flow | 36 |
| Pt 2: control of GI bleeding; follow-up, 6 mo | Pt 2: VWF/FVIII concentrate | ||||
| 1 (type 1 VWD) | 20 mg intramuscular every month | Control of GI bleeding (in association with propranolol 20 mg/3 times per day); follow-up, 8 mo | Desmopressin prophylaxis | Reduction of splanchnic and portal blood flow | 37 |
| Thalidomide | |||||
| 1 (type 2B VWD) | 150 mg/d | Control of GI bleeding; follow-up, 5 mo | Octreotide, discontinued for diarrhea | Antiangiogenic effect | 38 |
| 1 (type 2A) | 100 mg/d, increased to 150 mg/d for bleeding | Initial control of GI bleeding for 1 y, subsequent recurrence; follow-up, 22 mo | Desmopressin, tranexamic acid, VWF/FVIII concentrate | Antiangiogenic effect | 39 |
| 1 (type 2B) | 100 mg/d, decreased to 50 mg/d for fatigue | Control of GI bleeding (in association with estradiol/norethisterone, tranexamic acid), follow-up | VWF concentrate prophylaxis, recombinant activated FVII | Antiangiogenic effect | 40 |
| 1 (AVWS associated to IgG monoclonal gammopathy) | 50 mg/d | Control of GI bleeding; follow-up, 3 y | Desmopressin, VWF/FVIII concentrate, IV immunoglobulins, tranexamic acid, propranolol | Antiangiogenic effect | 41 |
| Lenalidomide | |||||
| 5 (3 type 3 VWD, 1 type 1 VWD, 1 type 2A VWD) | 5 mg/d for 3 wk on and 1 wk off, uptitrated to 10 and 15 mg/d if necessary (tapering to 2 wk on and 2 off when GI bleeding was obtained) | Control of GI bleeding; follow-up, 4-24 mo | Not reported | Antiangiogenic effect | 42 |
| 1 (AVWS associated to IgM monoclonal gammopathy) | 25 mg/d for 3 wk on and 1 wk off (decreased to 20 mg/d for 3 wk on and 1 wk off) | Control of GI bleeding; follow-up, 11 mo | Tranexamic acid, VWF/FVIII concentrate prophylaxis, plasma exchange, IV immunoglobulins, atorvastatin, octreotide, rituximab, and bendamustine | Antiangiogenic effect | 43 |
| Rituximab | |||||
| 2 (AVWS associated to monoclonal gammopathy) | Pt 1: 350 mg/m2 for 2 administrations | Not efficacious | Successful treatment with IV immunoglobulins | Anti-CD20, immunosuppressive effect | 44 |
| Pt 2: 350 mg/m2 for 4 administrations | |||||
| 1 (AVWS associated to systemic lupus erythematosus) | 375 mg/m2 biweekly in 2 doses | Control of GI bleeding and normalization of VWF levels | Steroid and cyclophosphamide | Anti-CD20, immunosuppressive effect | 45 |
| 1 (AVWS, characteristics not specified) | 375 mg/m2 weekly for 4 wk | Control of GI bleeding; follow-up, 5 mo | VWF concentrate, thalidomide; successful treatment with IV immunoglobulins | Anti-CD20, immunosuppressive effect | 46 |
Pt, patient.
Recurrent GI bleeding associated with type 1 VWD
Case 3
A 70-year-old woman with type 1 VWD (historic levels at 33 years: FVIII:C, 60 IU/dL; VWF:Ag, 35 IU/dL; VWF:RCo, 35 IU/dL; with a normal multimeric pattern, gene mutation p.C1927R) was admitted for hematemesis and anemia (hemoglobin, 7.3 g/dL; FVIII:C, 113%; VWF:Ag, 60%; VWF:RCo, 40%). Endoscopy revealed diffused inflammation of the gastric mucosa.
Medical history
The patient had experienced a previous episode of GI bleeding in her youth and another one 4 years ago.
Therapy
The patient received desmopressin (DDAVP), a proton-pump inhibitor, oral supplementation with iron, and folic acid.
Follow-up
The patient had recurrent GI bleeding (6 episodes in 6 months, 11 U of red blood cells transfused). After 6 months of follow-up, despite near normal VWF levels and a normal distribution of multimers, due to her repeated episodes of GI bleeding, prophylaxis with a plasma-derived VWF concentrate was started (Wilfactin, 40 IU VWF per kilogram twice per week, increased to 3 times per week after 6 months). Prophylaxis was carried out for 12 months without stopping GI bleeding (9 U of red blood cells were transfused). After this period, the patient was diagnosed with breast cancer. After surgery, she was treated with tamoxifen (20 mg per day); since its start, GI bleeding ceased and the patient received no more blood transfusions. The prophylaxis with the VWF concentrate was discontinued after GI bleeding stopped (1 month after beginning tamoxifen).
Discussion
GI bleeding due to angiodysplasia is not only typically associated with VWD types characterized by the loss of HMWMs, but it can also be present (albeit seldom) in patients with mild VWD and in healthy elderly subjects. In healthy individuals, angiodysplasia is associated with 4% to 7% of upper nonvariceal bleeding, 35% to 66% of small bowel occult bleeding, and 3% to 40% of colonic bleeding episodes.14 We cannot be sure that the GI bleeding in this patient was really triggered by the mild congenital VWD. The increasing levels with aging of VWF:RCo observed in the patient (affected by type 1 VWD) is also described in the Dutch cohort by Sanders et al. In this large group of patients, increasing levels of VWF activity were not associated with an improvement of the bleeding phenotype.15 Additionally, in our patient, the GI bleeding shortly preceded the diagnosis of breast cancer, which was not associated with a decrease of VWF levels (as we can see in cases of AVWS).
The role of prophylaxis with VWF concentrates in these patients is debatable and a different therapeutic approach may be more effective. For example, in our patient, the use of tamoxifen (driven by the presence of breast cancer) was rapidly effective in the control of GI bleeding. In The Netherlands, a trial is currently ongoing to evaluate the use of long-acting octreotide in the treatment of refractory anemia due to angiodysplasia in patients not affected by VWD (www.clinicaltrials.gov NCT02384122; accessed 9 April 2020).
AVWS associated with monoclonal gammopathy
Case 4
A 72-year-old man presented with repeated bleeding after tooth extractions. For this symptom, he was screened for a bleeding disorder and an AVWS associated with monoclonal gammopathy (immunoglobulin G [IgG] κ, 1.5 g/dL) was diagnosed (FVIII:C, 27 IU/dL; VWF:Ag, 23 IU/dL; VWF:RCo, <6 IU/dL; loss of HMWM). In the next 7 years, he had 5 episodes of GI bleeding. Because desmopressin and a VWF/FVIII concentrate failed to achieve a sustained response, he received high-dose IV immunoglobulins (1 g/kg for 2 days, split into 400 mg/kg per day for 5 days for feasibility reasons), reaching normal levels of FVIII and VWF after 3 days. However, FVIII and VWF returned to low baseline levels after 4 weeks.
At age 79 years, he was admitted into the hospital 5 times in 7 months with melena and anemia, requiring red blood cell transfusion and treatment with IV immunoglobulins. Video-capsule endoscopy revealed angiodysplasia in the jejunum. As rescue therapy, he was given 25 mg of lenalidomide per day for 21 days every 28 days for 6 cycles with no significant change in the GI bleeding (5 episodes in 6 months). Lenalidomide was therefore stopped and a reduced dose of IV immunoglobulins (400 mg/kg per day for 3 days every 4 weeks) was started, with no more acute episodes of GI bleeding. After 6 months, the dose was reduced to 400 mg/kg per day for 2 days every 4 weeks for another 6 months (no GI bleeding during this period) and eventually stopped. The patient is actually in follow-up, with normal hemoglobin levels but positive occult blood in the stools.
Discussion
Patients with AVWS may present with life-threatening GI bleeding that can be difficult to tackle because the use of VWF/FVIII concentrate may be hampered by the increased clearance of VWF. We shall focus herewith on those with monoclonal gammopathy because this group of patients may have very low levels of FVIII and VWF and recurrent GI bleeding.16 The use of high-dose IV immunoglobulins (1 g/kg for 2 days), evaluated in the context of a small prospective study (10 patients with AVWS associated with monoclonal component),17 was able to normalize FVIII and VWF levels in 8 of 10 patients (all with an IgG monoclonal component). However, the 2 patients with an IgM component failed to show a good response,17 at variance with a recent report of 2 patients.18 Thus, our current approach is to attempt this therapeutic approach even in patients with an IgM monoclonal component in cases of severe GI bleeding. The mechanism of action of this treatment is not clear: only in 1 of 2 patients did immunoglobulins dramatically reduce the VWF propeptide/antigen ratio (from 10.7 to 1.6), thus indicating a posttreatment normalization of VWF clearance.18 This therapeutic approach has no immediate effect and needs at least 24 to 48 hours before FVIII and VWF levels start to rise, but the effect may last for 2 to 4 weeks.17 These characteristics make immunoglobulins very useful in cases of scheduled surgery but less convenient in the frame of emergencies. In cases of life-threatening GI bleeding, also associating a VWF/FVIII concentrate, with close monitoring of FVIII and VWF levels owing to their accelerated clearance, may be necessary. Treatment with immunoglobulins can increase the half-life of VWF/FVIII concentrates even in those patients who only partially correct baseline levels of VWF. The use of high-dose IV immunoglobulins in the context of a prophylaxis regimen is not evidence based but was chosen in our patient because of the high number of recurrent bleeding episodes, in order to avoid hospitalization and acute GI bleeding. Despite their efficacy, high-dose IV immunoglobulins are not compatible with home treatment. As in congenital VWD, several rescue therapies were also reported for AVWS and GI bleeding (Table 1). The choice of a rescue therapy must carefully consider, in each patient, age, comorbidities, and possible side effects.
AVWS associated with heart valve defects
Case 5
A 37-year-old woman, affected by Cantú syndrome (a rare disorder characterized by congenital hypertrichosis, osteochondrodysplasia, cardiomegaly, and dysmorphism) and by a severe mitral valve insufficiency, came to our attention after a recent episode of heart failure before mitral valvuloplasty. AVWS with a mild reduction of VWF was diagnosed (FVIII:C, 84 IU/dL; VWF:Ag, 68 IU/dL; VWF:RCo, 38 IU/dL; loss of HMWM), associated with mild anemia and iron deficiency (hemoglobin, 11.5 g/dL; normal range,12-16 g/dL; ferritin, 5 μg/L; normal range, 15-150) and presence of occult blood in the stools. Before surgery, she received IV iron supplementation and desmopressin (1 dose, 0.3 μg/kg). Seven days later, surgery levels of FVIII and VWF were within the normal range (FVIII:C, 163 IU/dL; VWF:Ag, 130 IU/dL; VWF:RCo, 107 IU/dL).
Discussion
The association between GI bleeding and aortic stenosis in 10 elderly patients was first described by Heyde in 1958.19 It is now recognized that Heyde syndrome is caused by AVWS due to increased shear stress at the level of the aortic valve. In 2003, Batur et al20 showed increased prevalence of aortic stenosis (32%) in patients with angiodysplasia compared to those without (14%). These data were confirmed by an epidemiological study carried out in the 2011 US Nationwide Inpatient Sample database: from a total of 32 079 hospitalizations due to bleeding associated with GI telangiectasia, 7% had coexistent aortic valve disease (odds ratio, 2.37; 95% confidence interval, 2.10-2.66; adjusted for age and known risk factors); an association with mitral valve disease was not demonstrated.21
AVWS and GI bleeding also occur in patients receiving continuous-flow LVADs for the management of advanced heart failure (as a bridge to transplant therapy and also as destination therapy in the last few years).22 AVWS associated with LVADs was first shown by Geisen et al in 200823 in 7 patients with LVADs and elegantly confirmed by Kang et al24 in paired samples before and after LVAD implantation. AVWS associated with LVADs is an interesting model in which to study the mechanism of GI bleeding when there is a loss of HMWM. Indeed, elevated levels of angiopoietin-2 were shown in patients with LVADs,25 and plasma from patients with LVADs caused abnormal neoangiogenesis in vitro (reduced tubule length and migration).26
The prevalence of GI bleeding associated with continuous-flow LVADs has been estimated between 15% and 61%,27 and angiodysplasia is present in 30% to 60% of patients, with a significant burden of morbidity and health costs.22 In AVWS associated with heart valve defects or LVADs, at variance with autoimmune diseases or monoclonal gammopathies, VWF:RCo levels are generally normal or only mildly decreased in plasma but there is a consistent loss of HMWMs due to increased shear stress; bleeding can be severe and exacerbated by the use of antiplatelet drugs and/or anticoagulants. The loss of HMWMs can be suspected by the presence of a low RCo/Ag ratio, even when VWF:RCo is normal.
AVWS associated with valve disease can be cured by heart surgery. No evidence-based data are available on the use of desmopressin or VWF/FVIII concentrate before surgery in these patients.
Our strategy is based upon the evaluation of the degree of HMWM loss and the presence of bleeding symptoms in deciding whether a treatment with desmopressin or VWF/FVIII concentrate is useful. In these patients, there is generally no need to repeat the treatment because surgery is able to cure the AVWS.
In patients with acute GI bleeding and AVWS associated with continuous-flow LVADs, endoscopic examination is recommended together with the discontinuation of antiplatelet and anticoagulant therapy.28 The reinstitution of anticoagulant therapy after the resolution of the acute phase must be carefully monitored but it is the currently recommended approach in consideration of the thrombotic risk. In case there is recurrent GI bleeding, the guidelines recommend evaluating a possible reduction/discontinuation of antiplatelet/anticoagulant therapy and also considering a reduction of the device speed, with the goal of increasing pulsatility and ultimately reducing angiodysplasia development.28 In some patients with recurrent and severe GI bleeding, rescue therapies (danazol, octreotide, thalidomide) have been used and demonstrated to be useful in some case series or case-control studies.29 A randomized controlled study with a VWF concentrate prophylaxis in the first 3 months after LVAD implantation was stopped early because of poor enrollment (Prevention of Hemorrhage After Implantation of Mechanical Circulatory Support study, www.clinicaltrials.gov NCT02488525, accessed 22 December 2019).
General conclusions
GI bleeding may be a life-threatening condition that can arise both in congenital VWD and AVWS. Few data are available on the prevalence of this symptom, which is not common but is associated with high morbidity and costs. No data are available on the age of onset.
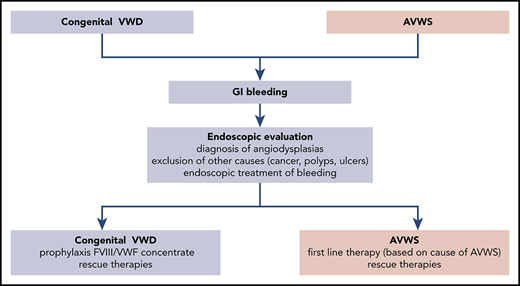
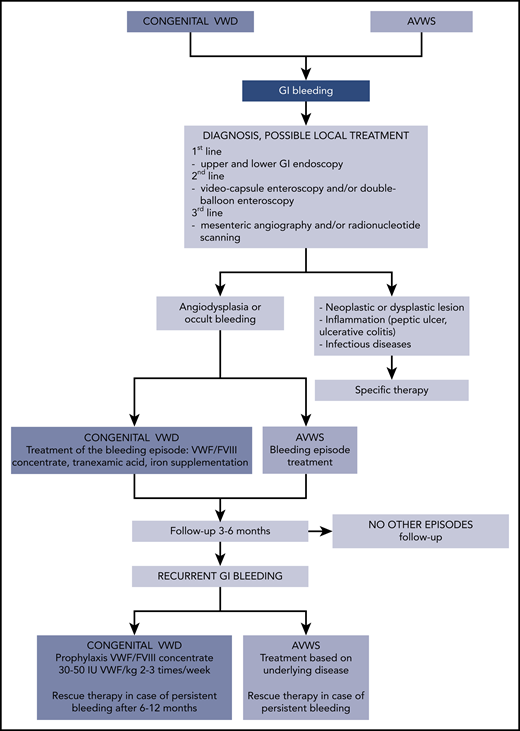
It is possible to hypothesize a period of latent bleeding at younger age when angiodysplasia starts to develop, followed by more evident and severe bleeding events when angiodysplasia worsens with aging or in association with other bleeding risk factors (gastritis, antiplatelet or anticoagulants drugs). The first-line therapy in congenital VWD is prophylaxis with VWF/FVIII or VWF concentrates, but the few available studies could not demonstrate its consistent efficacy, perhaps due to their relatively short follow-up. GI bleeding can be so severe and intractable that rescue therapies need to be associated in some patients, but data on their efficacy are very limited (case reports and case series with no long-term follow-up). In cases of GI bleeding and AVWS, the therapeutic choice is based on the control of the associated disease; but in cases of refractory bleeding, some second-line therapies have been described (Figure 1).
Flowchart of diagnosis, follow-up, and treatment of GI bleeding in congenital VWD and AVWS.
Flowchart of diagnosis, follow-up, and treatment of GI bleeding in congenital VWD and AVWS.
Acknowledgment
The authors thank P. M. Mannucci for helpful discussions and support.
Authorship
Contribution: E.B., S.M.S., and F.P. wrote the article.
Conflict-of-interest disclosure: F.P. has received honoraria for participating as a speaker at satellite symposia organized by Alnylam, Grifols, Kedrion, Roche, and Takeda, and reports participation in advisory boards for Bioverativ, Roche, Sanofi, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Eugenia Biguzzi, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Via Pace 9, 20122 Milan, Italy; e-mail: eugenia.biguzzi@policlinico.mi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal