In this issue of Blood, Kanayama et al have provided a novel solution to a problem that has long vexed the hematopoiesis field: how to identify hematopoietic stem and progenitor cell (HSPC) populations reliably, even under conditions of inflammation.1
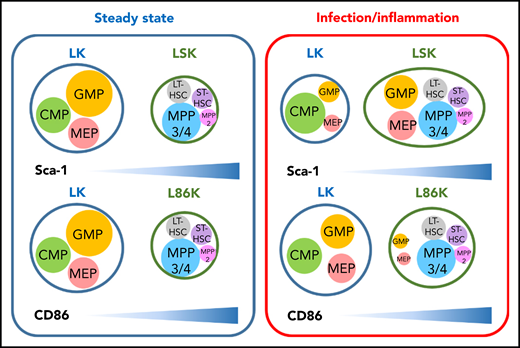
CD86 is a reliable marker to distinguish HSCs and MPPs from committed progenitors in inflammatory conditions. Immunophenotypes typically used to identify HSPCs have been determined largely in the context of steady-state hematopoiesis (left). However, Sca-1 expression is induced in a type I IFN-dependent manner on committed progenitors that typically do not express Sca-1, making them difficult to identify in inflammatory contexts. Replacing Sca-1 with CD86 allows efficient identification of committed progenitors, even in conditions associated with inflammation (right). The depicted sizes of HSPC populations in the LK, LSK, and L86K compartments indicate the relative frequencies of cells in each compartment in each context (steady state hematopoiesis or infection/inflammation), and is not intended to be indicative of absolute cell numbers. CMP, common myeloid progenitor; MPP, mulipotent progenitor; LT-HSC, long-term HSC; ST-HSC, short-term HSC.
CD86 is a reliable marker to distinguish HSCs and MPPs from committed progenitors in inflammatory conditions. Immunophenotypes typically used to identify HSPCs have been determined largely in the context of steady-state hematopoiesis (left). However, Sca-1 expression is induced in a type I IFN-dependent manner on committed progenitors that typically do not express Sca-1, making them difficult to identify in inflammatory contexts. Replacing Sca-1 with CD86 allows efficient identification of committed progenitors, even in conditions associated with inflammation (right). The depicted sizes of HSPC populations in the LK, LSK, and L86K compartments indicate the relative frequencies of cells in each compartment in each context (steady state hematopoiesis or infection/inflammation), and is not intended to be indicative of absolute cell numbers. CMP, common myeloid progenitor; MPP, mulipotent progenitor; LT-HSC, long-term HSC; ST-HSC, short-term HSC.
The hematopoietic system is hierarchically organized, with self-renewing, multipotent hematopoietic stem cells (HSCs) giving rise to downstream progenitors that exhibit progressively reduced self-renewal and estricted lineage capacity. These relationships have been extensively studied during steady-state hematopoiesis, and most are preserved during times of stress, even during infection or inflammation.2 Unfortunately, investigation of HSPCs has been limited by the inability to accurately identify and prospectively separate committed myeloid progenitor populations because Sca-1, a key marker used to identify these cells during steady-state hematopoiesis, is induced in response to inflammatory stimuli. Indeed, the authors confirmed that inflammatory stimuli induced Sca-1 expression in a type I interferon (IFN)–dependent manner, which confirms previous studies.3
By screening 180 surface molecules, Kanayama et al identified CD86 as an alternative marker to Sca-1 to identify HSPC populations. Although Sca-1 and CD86 show similar patterns of expression among Lin−c-Kit+ HSPCs at steady state, under conditions of inflammatory stress, including lipopolysaccharide (LPS) challenge, cecal ligation puncture, and polyinosinic-polycytidylic acid (polyI:C) treatment, Sca-1 expression was induced among Lin−c-Kit+ cells, resulting in a shift of granulocyte-macrophage progenitors (GMPs) and megakaryocyte-erythrocyte progenitors (MEPs) into the Lin−Sca-1+c-Kit+ (LSK) gate as demonstrated by in vitro clonogenic and differentiation assays. In contrast, CD86 retained more consistent expression levels among Lin−c-Kit+ HSPCs. These results were confirmed in vivo, when LSK cells from mice treated with LPS showed impaired reconstitution capacity, but Lin−c-Kit+CD86+ (L86K) cells showed comparable engraftment levels to naïve LSKs in the competitive transplantation setting. Together, these data definitively demonstrate that Lin−c-Kit+CD86− (LK) and Lin−c-Kit+CD86+ (L86K) cells more accurately capture classical HSPC populations under inflammatory stress (see figure). CD86 is not a perfect marker though, because 10% of myeloid progenitors acquired low levels of CD86 expression after LPS injection, causing a slight expansion of L86K cells. Nonetheless, the authors demonstrated that GMP and MEP upregulated Sca-1, and that these progenitors reduced the apparent frequency of CD34−CD150+LSK long-term HSCs after LPS injection. This finding stands in contrast to a previous study that showed that only the multipotent progenitor (MPP) gate, but not the HSC gate (Flk2−CD48−CD150+LSK), was contaminated with myeloid progenitors (MPs) in response to type I IFN administration.3
Enhanced granulocytic and monocytic differentiation under stress is well-described,2 but erythropoiesis may also be altered during inflammatory stress. Indeed, distinct stress erythroid progenitors derived from bone marrow (BM) short-term HSCs have been described.4 Now, using low CD86 expression to identify MPs, the investigators have shown that erythroid precursors also are mobilized after LPS stimulation, evidenced by increased numbers of MEPs, erythroblasts, and mature red blood cells (RBCs), as well as increased mature RBC egress from the BM to the periphery within hours post-LPS treatment. Moreover, the newly formed mature RBCs displayed unique characteristics, including increased hemoglobin content as well as resistance to apoptosis and hypo-osmolality. This does not seem to be merely the result of increased premature erythroid precursor mobilization because the RBCs showed a mature RBC immunophenotype. Thus, the authors speculated that the main value of stress erythropoiesis may be to generate a wave of erythrocytes that are resistant to infection- or inflammation-induced hemolysis and are resilient enough to maintain homeostasis until BM steady-state erythropoiesis restarts.5 This is certainly an intriguing model, but such a model requires additional investigation.
These findings are notable for their implications for prior investigations of HSC heterogenity. Previous reports described heterogeneous expression of CD86 among HSCs (CD48−CD150+LSK and CD34−Flk2−LSK), with CD86− HSCs expanding during aging or with chronic LPS treatment. These CD86− HSCs showed poor long-term reconstitution activity in transplant experiments as well as myeloid-biased differentiation compared with CD86+ HSCs.6 The Kanayama et al study suggests that the CD86− HSCs identified after LPS challenge may simply represent MPs contaminating the immunophenotypically defined HSC pool. Thus, it is possible that CD86high expression among LSK cells can help identify aged HSCs with the highest levels of self-renewal and balanced lineage output.
The ability to more accurately identify HSPCs during stress will likely promote exploration of several unresolved questions in hematopoiesis. Although the authors have tested CD86 in the context of experimental models of acute inflammation, it is important to determine whether CD86 can help resolve HSPC populations under other conditions associated with inflammation. For example, conditioning regimens such as irradiation create an inflammatory microenvironment into which newly transplanted HSPCs enter, but using CD86 may allow investigators to more accurately determine the fate of MP and HSC populations immediately after BM transplantation. Given that IFN-γ has been reported to cause impaired human HSC self-renewal and induce myeloid lineage bias,7 these studies also raise the question of whether isolation of human HSCs and committed myeloid progenitors under inflammatory conditions is compromised by changes in the expression of cell surface markers such as CD38, CD90, CD123, and CD45RA.8 Studies of human hematopoiesis under inflammatory stress are few, and thus we anticipate such studies to be the subject of future investigations.
Conflict-of-interest disclosure: The authors declare no competing financial interests.