TO THE EDITOR:
Complete remissions (CRs) are infrequent in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib,1 and, unlike in patients treated with chemo-immunotherapy2 or venetoclax,3 it remains uncertain whether there is any correlation between depth of remission and outcome in these patients. In particular, minimal residual disease (MRD) eradication is very rarely achieved with the use of single-agent ibrutinib treatment.1
Also, factors predictive of the depth of remission in CLL patients treated with ibrutinib are lacking. In this regard, the bone marrow (BM) generally constitutes the main site of residual disease during treatment with ibrutinib,1 and cells within the BM microenvironment, such as macrophages, may support the survival of CLL cells during therapy.4
We prospectively assessed responses to therapy, independent of MRD eradication status, in 208 patients with previously untreated or relapsed refractory CLL, treated on a randomized phase 2 trial of ibrutinib (alone or in combination with rituximab), at The University of Texas MD Anderson Cancer Center, from December 2013 through October 2017 (registered at clinicaltrials.gov as #NCT02007044).5 This study was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board at MD Anderson Cancer Center. Responses were evaluated according to the 2008 International Workshop on Chronic Lymphocytic Leukemia criteria,6 with the exception that lymphocytosis was not the sole criterion for disease progression. Categorical variables were compared using the χ2 or Fisher’s exact test, as appropriate. Progression-free survival (PFS) was defined as the time from start of treatment to progression, death, or last follow-up, and overall survival (OS) as the time from start of treatment to death or last follow-up. Survival curves and median time to response were calculated using the method of Kaplan and Meier, and univariate comparisons were made with the log-rank test. All tests were 2 sided, and results were considered significant if P ≤ .05.
BM tumor-associated macrophages (TAMs) were stained with the following immunohistochemistry (IHC) markers: CD68 (general macrophage marker, including both antitumoral [M1] and protumoral [M2] phenotype), and CD163, CSF1-R, PDL-1 (clone E1L3N), and arginase 1 (protumoral or M2 macrophage markers); normal BM cells were also evaluated as controls. IHC slides were digitally scanned with the Aperio AT2 at ×20 and analyzed using digital image-analysis HALO software (Indica Laboratory), and cell density was reported as the number of positive cells per square millimeter.
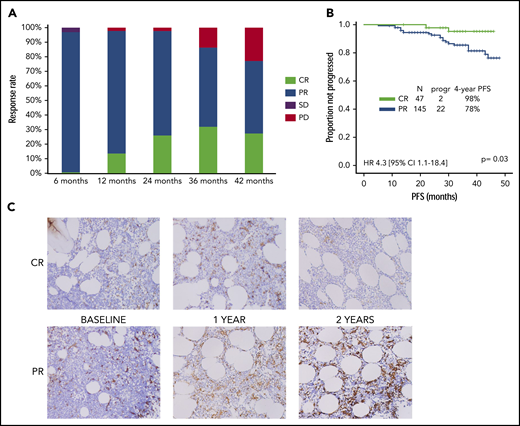
Two hundred eight patients were included in the study: response was evaluable in 194, and 14 stopped treatment before the first response assessment. Overall response rate was achieved in 192 (99%) and CR in 47 (24%) of the 194 patients (6 were MRD− [3%]), after a median time of 10 months (range, 3-45 months) and 21 months (range, 5-45 months), respectively. Response rates at each time point are shown in Figure 1A.
Response rate, PFS, and TAM phenotype by quality of response. (A) Response rates at each time point. (B) PFS by CR vs PR (data confirmed in a landmark analysis at 21 months). (C) CD163+ TAM density by CR vs PR. CI, confidence interval; HR, hazard ratio; PD, progressive disease; SD, stable disease.
Response rate, PFS, and TAM phenotype by quality of response. (A) Response rates at each time point. (B) PFS by CR vs PR (data confirmed in a landmark analysis at 21 months). (C) CD163+ TAM density by CR vs PR. CI, confidence interval; HR, hazard ratio; PD, progressive disease; SD, stable disease.
Baseline characteristics of the 47 patients who achieved CR were compared with those of 145 patients who achieved partial response (PR), and no significant association was observed in univariate analyses (Table 1). Seventy-one of 208 patients discontinued treatment after a median treatment duration of 12 months (range, 1-45 months); toxicity was the most common cause of discontinuation among patients with CR (71%) or PR (52%). Median duration of treatment among patients who discontinued ibrutinib for reasons other than progression or consolidation was 13 months, and no statistically significant difference was observed when comparing patients who achieved CR to those who achieved PR (15 vs 11 months; P = .13). After a median follow-up of 4 years, median PFS for all 208 patients was not reached, and 28 patients (13%) progressed and/or died. The association between all baseline characteristics shown in Table 1, depth of response (CR vs PR), and PFS was evaluated. Using univariate analysis, the only 3 factors significantly associated with a longer PFS were absence of complex karyotype (4-year PFS, 80% vs 40%; P = .05), absence of deletion 17p/TP53 mutation (4-year PFS, 90% vs 70%; P = .03), and achievement of CR (98% vs 78%; hazard ratio, 4.3; 95% confidence interval, 1.1-18.4; P = .03; Figure 1B). The latter was also maintained after adjustment for deletion 17p/TP53 status (P = .03). The association between achievement of CR and PFS was also confirmed in a landmark analysis at 21 months (2-year PFS 98% vs 78%; hazard ratio, 5.8; 95% confidence interval, 1.1-44.6; P = .05), including 141 patients, all receiving ibrutinib at the time of landmarking and with a median follow-up of 2 years.
Factors associated with achievement of CR
| . | CR (n = 47) n, (%) . | PR (n = 145) n, (%) . | P . |
|---|---|---|---|
| Age <65 y | 23 (49) | 71 (49) | .99 |
| Age ≥65 y | 24 (51) | 74 (51) | |
| Females | 17 (36) | 40 (28) | .28 |
| Males | 30 (64) | 105 (72) | |
| Previously untreated | 9 (19) | 17 (12) | .22 |
| Previously treated | 38 (81) | 128 (88) | |
| Rai stage 0-II | 32 (67) | 89 (61) | .49 |
| Rai stage III-IV | 15 (33) | 56 (39) | |
| Nonbulky LNs | 43 (91) | 127 (88) | .60 |
| Bulky LNs (≥5 cm) | 4 (9) | 18 (12) | |
| B2M <4 mg/dL | 26 (55) | 82 (59) | .73 |
| B2M ≥4 mg/dL | 21 (45) | 57 (41) | |
| No deletion of 17p/TP53 mutation | 27 (57) | 88 (62) | .61 |
| Deletion of 17p/TP53 mutation | 20 (43) | 54 (38) | |
| TP53 mutated | 21 (70) | 65 (63) | .52 |
| TP53 not mutated | 9 (30) | 38 (37) | |
| Karyotype, noncomplex | 15 (75) | 45 (61) | .30 |
| Karyotype, complex | 5 (25) | 29 (39) | |
| IGHV mutated | 9 (23) | 35 (30) | .42 |
| IGHV not mutated | 31 (77) | 83 (70) | |
| Ibrutinib | 26 (55) | 70 (48) | .50 |
| Ibrutinib+rituximab | 21 (45) | 75 (52) |
| . | CR (n = 47) n, (%) . | PR (n = 145) n, (%) . | P . |
|---|---|---|---|
| Age <65 y | 23 (49) | 71 (49) | .99 |
| Age ≥65 y | 24 (51) | 74 (51) | |
| Females | 17 (36) | 40 (28) | .28 |
| Males | 30 (64) | 105 (72) | |
| Previously untreated | 9 (19) | 17 (12) | .22 |
| Previously treated | 38 (81) | 128 (88) | |
| Rai stage 0-II | 32 (67) | 89 (61) | .49 |
| Rai stage III-IV | 15 (33) | 56 (39) | |
| Nonbulky LNs | 43 (91) | 127 (88) | .60 |
| Bulky LNs (≥5 cm) | 4 (9) | 18 (12) | |
| B2M <4 mg/dL | 26 (55) | 82 (59) | .73 |
| B2M ≥4 mg/dL | 21 (45) | 57 (41) | |
| No deletion of 17p/TP53 mutation | 27 (57) | 88 (62) | .61 |
| Deletion of 17p/TP53 mutation | 20 (43) | 54 (38) | |
| TP53 mutated | 21 (70) | 65 (63) | .52 |
| TP53 not mutated | 9 (30) | 38 (37) | |
| Karyotype, noncomplex | 15 (75) | 45 (61) | .30 |
| Karyotype, complex | 5 (25) | 29 (39) | |
| IGHV mutated | 9 (23) | 35 (30) | .42 |
| IGHV not mutated | 31 (77) | 83 (70) | |
| Ibrutinib | 26 (55) | 70 (48) | .50 |
| Ibrutinib+rituximab | 21 (45) | 75 (52) |
N = 192 patients. Fluorescence in situ hybridization data were not available for 6 patients, karyotype for 98 patients, and IGHV mutational status for 36 patients.
B2M, B2 microglobulin; LN, lymph node.
The phenotype of BM TAMs was characterized by IHC on 32 serial BM samples derived from a subgroup of 12 previously untreated patients receiving single-agent ibrutinib treatment for >2 years, 9 of whom had achieved PR and 3 CR at the most recent follow-up. CSF1-R, PDL-1, and arginase-1 were negative in all patients at all time points. The median (± standard error) density of CD68+ macrophages was similar in patients with PR, compared with those with CR at baseline (152 ± 25 cells/mm2 vs 136 ± 54 cells/mm2; P = .54) and after 2 years on treatment (146 ± 77 cells/mm2 vs 141 ± 82 cells/mm2; P = .80), and higher at 1 year (324 ± 89 cells/mm2 vs 91 ± 62 cells/mm2; P = .11). The median density of CD163+ macrophages (M2) was higher in patients with PR than in those with CR at baseline (313 ± 47 cells/mm2 vs 170 ± 70 cells/mm2; P = .22), 1 year (415 ± 77 cells/mm2 vs 108 ± 33 cells/mm2; P = .02), and 2 years (231 ± 74 cells/mm2 vs 83 ± 37 cells/mm2; P = .44; Figure 1C).
To our knowledge, this is the first study to demonstrate that achievement of CR is a desirable end point for patients with CLL treated with ibrutinib. Sigmund et al7 previously reported an association between achievement of a very good PR (defined as residual lymphadenopathy at 1 year <3 cm) and prolonged PFS, but failed to observe an association with achievement of CR. Of interest, in that study, response at 12 months, as opposed to best response, was used for the analysis. In this regard, a recent study,8 as well as our study, showed that patients can have an improvement in quality of response up to 2 years after initiation of treatment with ibrutinib, most likely explaining the lack of an observed association in the study by Sigmund et al between CR at 1 year and prolonged PFS.
In our analysis, none of the patient’s baseline characteristics, including traditional prognostic factors, was associated with achievement of CR in this population. O’Brien et al8 recently reported a retrospective analysis of pooled data from 2 clinical trials and observed that previously untreated patients and those without bulky lymphadenopathy (≥5 cm) were more likely to achieve CR when treated with ibrutinib alone. Only 26 previously untreated patients were evaluable for analysis of response in our study, limiting statistical comparisons. In addition, only 22 patients with bulky lymphadenopathy were enrolled in our study, again impairing the statistical significance of comparisons.
Finally, in this study, a higher density of CD163+ cells was observed in patients treated with ibrutinib who did not achieve CR, although statistical significance was limited by sample size. Although its specificity is limited, CD163 is typically used to identify protumoral (M2) TAMs.9 Preclinical studies have shown that ibrutinib may impair the phagocytic capacity of TAMs and promote macrophage polarization to an M2 phenotype.10,11 In our analysis, when we used serial BM samples obtained from patients with CLL before and during ibrutinib therapy, the density of M2 TAMs was higher in patients with PR than in those with CR, even before the initiation of treatment. This result suggests that a high density of BM M2 TAMs may be a prognostic factor, rather than a consequence of treatment, and agents that target these cells may improve the depth of response to ibrutinib.
We acknowledge major limitations of this analysis. First, the original clinical trial was not statistically powered to analyze the association between achievement of CR and PFS. Second, correlative analysis was performed on a small sample size, using IHC only, and no confirmatory functional studies were performed.
Additional studies aimed at further characterizing the role of TAMs in the quality of response obtained with the use of ibrutinib may provide the preclinical rationale for future more effective combination strategies.
Acknowledgments
This work was supported in part by a National Institutes of Health (NIH)/National Cancer Institute (NCI) support grant (CA016672) to the MD Anderson Cancer Center and by the Conquer Cancer Foundation/ASCO Young Investigator Award.
Authorship
Contribution: P.S. designed the study, analyzed data, and wrote the paper; M.J.K., W.G.W., A.F., H.K., Z.E., N.J., and P.A.T. provided clinical care to patients and coauthored the paper; M.S. and E.K. collected clinical data and coauthored the paper; E.J.S., L.M.S.S., D.E.D., and I.I.W. performed IHC staining and analysis and coauthored the paper; J.A.B. designed the study, analyzed the data, provided clinical care to patients, and wrote the paper.
Conflict-of-interest disclosure: J.A.B. and N.J. have received research funding from Pharmacyclics and are consultants for Janssen Pharmaceuticals. N.J. and P.A.T. have served as consultants for AbbVie and Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Paolo Strati, Department of Lymphoma and Myeloma, Division of Cancer Medicine, MD Anderson Cancer Center, The University of Texas, Unit 428, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: pstrati@mdanderson.org.