TO THE EDITOR:
As the COVID-19 pandemic threatens to cause critical shortages in the blood supply, strategies to decrease blood utilization are necessary to ensure adequate blood availability. For patients with sickle cell anemia (SCA) who have suffered an overt stroke, continued monthly red blood cell (RBC) transfusion provides the greatest protection from secondary strokes compared with other disease-modifying therapies. Without chronic transfusion, these patients’ risk of recurrent stroke is >50%.1 Although treatment with hydroxyurea also provides some cerebrovascular benefits for patients with SCA,2-10 chronic transfusion offers better stroke protection. Based on the results of the Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) clinical trial, patients with a history of stroke ideally should not transition to hydroxyurea-only treatment, because this may increase their risk of stroke.11 The American Society of Hematology 2020 guidelines for sickle cell disease explicitly warn that “hydroxyurea therapy is an inferior alternative to regular blood transfusion for secondary stroke prevention.”12
Given the importance of social distancing with the COVID-19 pandemic, routine medical visits are appropriately being postponed or converted to virtual visits. However, for patients with SCA and a history of stroke, the risk of recurrent stroke due to abrupt cessation of regular transfusions is likely greater than the risk of infection from attending clinic. When considering whether transfusion practices should be changed during this time, the American Society of Hematology recommends an individualized assessment of patient risk, as well as an evaluation of local blood availability.13 Recently, DeBaun has suggested immediately starting low-dose hydroxyurea for all children with SCA receiving chronic transfusion for stroke prevention.14 As noted, low-dose hydroxyurea can be safely administered with minimal laboratory surveillance, a clear advantage during this challenging time; however, dose-escalated hydroxyurea appears to provide superior laboratory and clinical benefits.15-17 We agree with DeBaun that starting hydroxyurea now for patients on chronic transfusion is reasonable, but instead propose adding dose-escalated hydroxyurea based on the preliminary results of our active clinical trial of combination hydroxyurea and chronic transfusion (NCT03644953). In sharing this work, our goal is to help inform clinicians considering the use of hydroxyurea for patients with SCA on chronic transfusion during the current pandemic as part of a blood-conservation strategy.
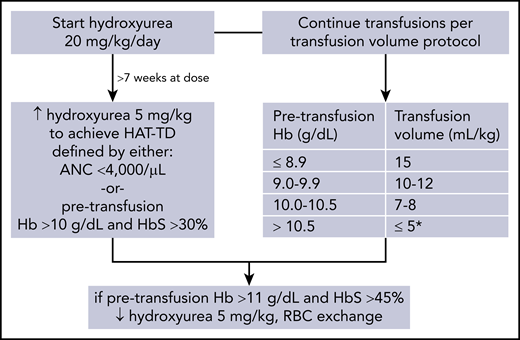
A few case series have reported potential clinical benefits of combination hydroxyurea and chronic transfusion therapy for SCA,18,19 but combination therapy has not been rigorously studied. Because progression of cerebrovascular disease is known to occur in patients on chronic transfusion,20 combination hydroxyurea and transfusion therapy may offer superior stroke protection. We designed a single-arm clinical trial of combination therapy in 15 patients to primarily evaluate its feasibility, as well as investigate its potential benefit in decreasing transfusion requirements as a prespecified secondary outcome. The Children’s National Hospital’s Institutional Review Board approved this study, and consent was obtained from all participants. Patients with hemoglobin (Hb) SS or Sβ0 thalassemia receiving simple chronic transfusions for stroke prevention were started on hydroxyurea, 20 mg/kg per day. The hydroxyurea dose was increased by 5 mg/kg per day after >7 weeks of a dose to achieve a hydroxyurea and transfusion target dose (HAT-TD) defined by the absolute neutrophil count (ANC) or the pretransfusion Hb and Hb S percentage (Figure 1). During this time, patients continued simple chronic transfusion per the same transfusion protocol to achieve a target posttransfusion Hb of 11.5 to 12.0 g/dL (Figure 1). One potential problem associated with combination therapy is that hydroxyurea increases pretransfusion Hb levels, resulting in smaller RBC transfusion volumes that, in turn, could cause the pretransfusion Hb S to increase to >45%. Given this concern, patients were monitored closely to ensure a pretransfusion Hb <11.0 g/dL and Hb S <45%, and this was designated an adverse event a priori. Hydroxyurea was held for neutropenia (ANC < 1.0 × 109/L) or thrombocytopenia (platelet count <80 × 109/L); it was restarted when counts recovered.
Hydroxyurea and transfusion management protocol. *Consider postponing transfusion by 1 week if recent Hb S <35%.
Hydroxyurea and transfusion management protocol. *Consider postponing transfusion by 1 week if recent Hb S <35%.
To date, we have enrolled 14 patients (aged 5-19 years), with a median time on study of 45 weeks (range, 15-77 weeks). Combination hydroxyurea (median dose, 25 mg/kg per day for active patients) and chronic transfusion has generally been well tolerated. All of these patients are on concurrent chelation therapy, but no overlapping toxicities have been observed. No patient receiving combination therapy has developed a pretransfusion Hb >11.0 g/dL and Hb S >45%. No patient has had a new stroke or neurologic event. One serious adverse event, splenic sequestration (nadir Hb, 7.2 with Hb S, 39.4%) in a patient with baseline splenomegaly, was possibly attributable to combination therapy; however, this patient resumed hydroxyurea after recovery from this acute illness and has had no further events.
Two patients have completed the study, which is defined as 1 year of combination treatment with hydroxyurea after reaching HAT-TD. HAT-TD was achieved for Patient02 after 19 weeks at a hydroxyurea dose of 30 mg/kg per day and for Patient06 after 17 weeks at a dose of 25 mg/kg per day. Table 1 compares their transfusion burden and pretransfusion laboratory parameters in the year prior to study enrollment and the year after achieving HAT-TD. Although both patients had an increase in their Hb S percentage, this occurred in the setting of an increased percentage of Hb F and less ongoing hemolysis, as demonstrated by improved pretransfusion Hb and lactate dehydrogenase values. Although neither patient experienced decreased transfusion frequency, both received decreased weight-based volumes of RBCs at each transfusion. The clinical significance of an ∼30-mL/kg reduction in RBCs transfused per year has great importance when considering universal and systemic blood shortages. At our institution we support 60 patients with SCA on chronic transfusion, so this reduction in volume per patient (assuming an average patient weight of 40 kg) would decrease our RBC transfusion need by an estimated 72 000 mL or 240 RBC units annually.
Comparison of transfusion burden and pretransfusion laboratory parameters before and after the addition of hydroxyurea to chronic transfusion
| . | 1 y before start of hydroxyurea . | 1 y after HAT-TD . | Change . | P* . |
|---|---|---|---|---|
| Total RBC volume, mL | ||||
| Patient02 | 6087 | 5995 | −92 | |
| Patient06 | 3887 | 3409 | −478 | |
| Total RBC volume/weight, mL/kg | ||||
| Patient02 | 268.3 | 234.1 | −34.2 | |
| Patient06 | 155.6 | 126.3 | −29.3 | |
| Transfusion events, n | ||||
| Patient02 | 24 | 25 | +1 | |
| Patient06 | 14 | 14 | 0 | |
| Hb S, median (IQR), % | ||||
| Patient02 | 16.4 (11.4-23.5) | 25 (20.6-29.7) | +8.6 | .001 |
| Patient06 | 28.1 (22.2-35.5) | 44 (33.9-49.1) | +15.9 | .017 |
| Hb F, median (IQR), % | ||||
| Patient02 | 2.9 (2.4-4.4) | 16.1 (14.1-17.6) | +13.2 | <.0001 |
| Patient06 | 1.3 (0.5-2.4) | 13.1 (9.6-16.1) | +11.8 | <.0001 |
| Total Hb, median (IQR), g/dL | ||||
| Patient02 | 8.9 (8.6-9.4) | 9.4 (9.1-10.1) | +0.5 | .0006 |
| Patient06 | 8.1 (7.8-8.5) | 9.5 (8.5-10.0) | +1.4 | .001 |
| LDH, median (IQR), unit/L | ||||
| Patient02 | 842 (701-956) | 531 (383-779) | −311 | .0005 |
| Patient06 | 976 (869-1289) | 521 (505-640) | −455 | <.0001 |
| . | 1 y before start of hydroxyurea . | 1 y after HAT-TD . | Change . | P* . |
|---|---|---|---|---|
| Total RBC volume, mL | ||||
| Patient02 | 6087 | 5995 | −92 | |
| Patient06 | 3887 | 3409 | −478 | |
| Total RBC volume/weight, mL/kg | ||||
| Patient02 | 268.3 | 234.1 | −34.2 | |
| Patient06 | 155.6 | 126.3 | −29.3 | |
| Transfusion events, n | ||||
| Patient02 | 24 | 25 | +1 | |
| Patient06 | 14 | 14 | 0 | |
| Hb S, median (IQR), % | ||||
| Patient02 | 16.4 (11.4-23.5) | 25 (20.6-29.7) | +8.6 | .001 |
| Patient06 | 28.1 (22.2-35.5) | 44 (33.9-49.1) | +15.9 | .017 |
| Hb F, median (IQR), % | ||||
| Patient02 | 2.9 (2.4-4.4) | 16.1 (14.1-17.6) | +13.2 | <.0001 |
| Patient06 | 1.3 (0.5-2.4) | 13.1 (9.6-16.1) | +11.8 | <.0001 |
| Total Hb, median (IQR), g/dL | ||||
| Patient02 | 8.9 (8.6-9.4) | 9.4 (9.1-10.1) | +0.5 | .0006 |
| Patient06 | 8.1 (7.8-8.5) | 9.5 (8.5-10.0) | +1.4 | .001 |
| LDH, median (IQR), unit/L | ||||
| Patient02 | 842 (701-956) | 531 (383-779) | −311 | .0005 |
| Patient06 | 976 (869-1289) | 521 (505-640) | −455 | <.0001 |
IQR, interquartile range, LDH, lactate dehydrogenase.
Mann-Whitney test, before hydroxyurea vs after hydroxyurea at target dose.
Continued clinical research on this topic is needed to better inform optimal chronic transfusion management and outcomes for SCA. Nonetheless, with the potential for the blood supply to be limited for the indefinite future as a result of COVID-19, adding dose-escalated hydroxyurea to chronic transfusion per our study protocol can be considered. We also advocate for the importance of blood donation during this time, particularly among individuals of African descent,21 so that patients with SCA and history of stroke can continue to benefit from regular transfusions.
Data sharing requests can be sent via e-mail to the corresponding author.
Acknowledgments
This work was supported by the National Blood Foundation Scientific Research Grants Program and the Clinical and Translational Science Institute at Children’s National Hospital (R.S.N.).
Authorship
Contribution: R.S.N., N.L.C.L., and J.W. designed the clinical trial; B.F. and J.W. provided clinical care to participants; S.M. coordinated the clinical trial and collected data; and all authors wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Sheppard Nickel, Children’s National Hospital, 111 Michigan Ave NW, Washington, DC 20010; e-mail: rnickel@childrensnational.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal