In this issue of Blood, Morodomi et al advance our understanding of the mechanisms involved in antibody-mediated immune thrombocytopenia (ITP).1
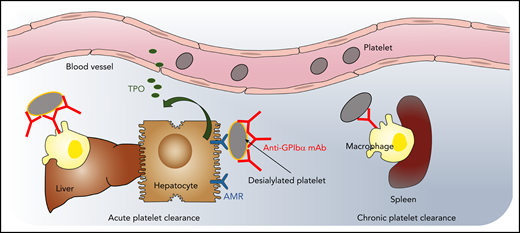
Model of acute and chronic anti-GPIbα antibody–induced platelet clearance. High-dose anti-GPIbα mAb injection opsonizes platelets, leading to platelet activation and aggregation, followed by a rapid clearance from the circulation by liver macrophages and hepatocytes via the AMR (ie, acute antibody-mediated platelet clearance). Hepatic platelet clearance induces rapid thrombopoietin (TPO) production. In contrast, low-dose subcutaneous injections lead to a gradual decrease in circulating platelet count (ie, mimicking chronic thrombocytopenia). After low-dose mAb, platelet clearance is only observed in the spleen by macrophages, and TPO levels remain unaltered. Illustration by Leonardo Rivadeneyra, Versiti Translational Glycomics Center.
Model of acute and chronic anti-GPIbα antibody–induced platelet clearance. High-dose anti-GPIbα mAb injection opsonizes platelets, leading to platelet activation and aggregation, followed by a rapid clearance from the circulation by liver macrophages and hepatocytes via the AMR (ie, acute antibody-mediated platelet clearance). Hepatic platelet clearance induces rapid thrombopoietin (TPO) production. In contrast, low-dose subcutaneous injections lead to a gradual decrease in circulating platelet count (ie, mimicking chronic thrombocytopenia). After low-dose mAb, platelet clearance is only observed in the spleen by macrophages, and TPO levels remain unaltered. Illustration by Leonardo Rivadeneyra, Versiti Translational Glycomics Center.
ITP is an autoimmune disease characterized by isolated thrombocytopenia with platelet counts <100 000 per cubic millimeter where other causes of thrombocytopenia have been excluded. ITP is idiopathic in 80% of cases.2 In 20% of cases, ITP is secondary to other illnesses, most commonly acute infections and chronic inflammatory processes, such as autoimmune and rheumatologic conditions; 1% to 5% of patients with chronic lymphocytic leukemia also develop ITP.2,3 The incidence of ITP in adults ranges from 2 to 4 cases per 100 000 per year, with 2 peaks in age: first between 20 and 30 years of age, with a slight female predominance, and a larger peak after 60 years of age affecting men and women equally.2,3 At presentation, patients with ITP may be asymptomatic, have mild mucocutaneous bleeding, or, in ∼5%, have life-threatening bleeding, such as intracranial hemorrhage.3 However, patients with ITP often report other symptoms, such as fatigue, and have reduced health-related quality of life.3 Paradoxically, the risk of venous thromboembolism is higher in ITP patients compared with the general population, complicating the management of venous thromboembolism, given the associated bleeding risk.3
The pathophysiology of ITP is complex and incompletely understood. The conventional explanation is that platelets with autoantibodies bound to their surface are prematurely destroyed in the spleen, liver, or both through interaction with Fcγ receptors.4 Autoantibodies can also induce complement-mediated5 or desialylation-induced destruction of platelets,6,7 as well as inhibit megakaryocyte function. Aged desialylated platelets are cleared via the hepatic Ashwell-Morell receptor (AMR).8 Recent findings support the notion that opsonization of platelets with anti-GPIbα antibodies activates the platelets, resulting in the translocation of neuraminidase-1 to the surface, where it desialylates the platelets, thereby leading to Fc-independent hepatic clearance via the hepatic AMR.6 Despite these elegant studies on specific aspects of ITP, the pathophysiology of the overall disorder remains an enigma.
The study by Morodomi et al investigates 2 different mouse models of ITP that use rat anti-mouse GPIbα monoclonal antibody (mAb) 5A7 to induce thrombocytopenia (see figure). To induce platelet depletion, in 1 model, a single high dose (2 mg/kg) of mAb 5A7 was injected IV. In the other model, a low dose of mAb 5A7 (0.08-0.16 mg/kg) was administered subcutaneously every 3 days. The high-dose mAb injection opsonized platelets, leading to platelet activation and aggregation, followed by rapid clearance from the circulation in the liver (ie, acute antibody-mediated platelet clearance). This rapid platelet clearance was associated with increased platelet activation and desialylation, leading to clearance via liver-resident macrophages and hepatic AMR, followed by rapid TPO messenger RNA upregulation in hepatocytes. In contrast, low-dose subcutaneous injections led to a gradual decrease in circulating platelet counts (ie, mimicking chronic thrombocytopenia). In the low-dose model, platelet clearance was only observed in the spleen by macrophages; TPO levels remained unaltered. In both mouse models, the 5A7 antibody was found on the surface and inside bone marrow megakaryocytes. However, only chronic 5A7 administration reduced GPIbα surface expression on circulating platelets, perhaps explaining why hepatic TPO is not upregulated in ITP.9
This study presents several novel findings. (1) The site of platelet clearance is dependent not only on the specificity of the antiplatelet antibody but also on the injection route and circulating concentrations. (2) High antibody concentrations induce platelet activation/desialylation and aggregation, leading to platelet aggregate removal by liver-resident macrophages and desialylated platelet removal by hepatocytes. (3) Repeated mAb injections and chronic thrombocytopenia opsonize platelets, leading to clearance via splenic macrophages. Hence, when generating ITP mouse models, the frequency and mode of antibody administration and antibody concentrations have to be taken into account when interpreting platelet clearance mechanisms.
It is tempting to speculate that in humans with acute antibody-induced thrombocytopenia, platelets are cleared via a different mechanism than in chronic thrombocytopenia. However, antiplatelet antibodies are not detected in up to 50% of patients with ITP, pointing to additional mechanisms of platelet destruction. Abnormalities in T cells have been described, including skewing of T helper (Th) cells toward Th1 and Th17 phenotypes4 and a reduction in regulatory T cells.4 A few studies also suggest that CD8+ T cells, specifically cytotoxic CD8+ T cells, enhance platelet clearance through phagocytosis by splenic macrophages or dendritic cells and by induction of platelet apoptosis. Bone marrow–resident CD8+ T cells also target megakaryocytes and inhibit thrombopoiesis.4 The study by Morodomi et al does not address whether antibody injections, specifically via the subcutaneous route, affect other immune-modulatory cells.
Recent data also suggest that liver macrophage lectins clear desialylated platelets via macrophage galactose lectins and likely assist hepatocytes to phagocytose desialylated platelets.10 Whether clearance of platelet aggregates by macrophages affects hepatocyte-mediated platelet clearance induced by antibody injections needs further clarification. Whether antibodies induce desialylation of megakaryocytes and activate bone marrow–resident immune cells, thereby modulating thrombopoiesis, remains unclear. The Morodomi et al study furthers our understanding of antibody-mediated platelet clearance and shows the need for additional mechanistic studies of ITP.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal