In the this issue of Blood, Schöder et al report the superior predictive value of change in maximum standardized uptake (ΔSUVmax) compared with Deauville score (DS) in interim positron emission tomography (i-PET) evaluation in 158 diffuse large B-cell lymphoma (DLBCL) patients treated with immunochemotherapy enrolled in the Alliance/CALGB 50303 trial.1
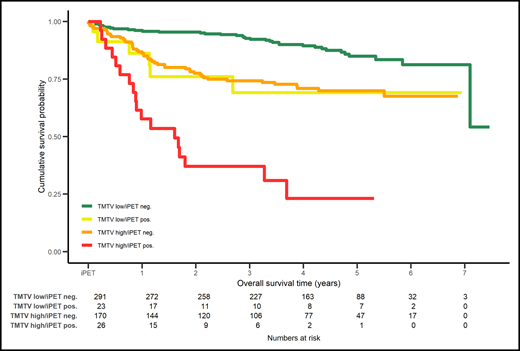
Combination of PET2 (positive or negative) assessed with ΔSUVmax with the baseline total metabolic tumor volume (TMTV) low or high in DLBCL patients enrolled in the PETAL study stratifies 4 groups of patients with different overall survival. neg., negative; pos., positive. Reprinted from European Journal of Cancer. 2020: 124, Schmitz C, Hüttmann A, Müller SP, et al, Dynamic risk assessment based on positron emission tomography scanning in diffuse large B-cell lymphoma: post-hoc analysis from the PETAL trial, pages 25-36. Copyright 2020, with permission from Elsevier.
Combination of PET2 (positive or negative) assessed with ΔSUVmax with the baseline total metabolic tumor volume (TMTV) low or high in DLBCL patients enrolled in the PETAL study stratifies 4 groups of patients with different overall survival. neg., negative; pos., positive. Reprinted from European Journal of Cancer. 2020: 124, Schmitz C, Hüttmann A, Müller SP, et al, Dynamic risk assessment based on positron emission tomography scanning in diffuse large B-cell lymphoma: post-hoc analysis from the PETAL trial, pages 25-36. Copyright 2020, with permission from Elsevier.
This imaging substudy compared the predictive value of i-PET performed after 2 cycles (PET2) and at the end of treatment (EoT PET) when utilizing the visual DS or a dynamic semiquantitative analysis (ΔSUVmax). A reproducible method for an early assessment of the chemosensitivity is critical in DLBCL to identify high risk patients and guide therapy (ie, response-adapted treatment). The study demonstrated that only the PET2 ΔSUVmax predicted progression-free survival (PFS) and overall survival (OS). A ΔSUVmax cutoff of ≥66% can predict survival with PET2 and both survival and PFS with EoTPET.2 By contrast, DS reading did not prove predictive of outcome at either the interim or EoT time points. ΔSUVmax proved superior to DS in predicting treatment outcome in DLBCL in an international retrospective multicenter analysis of 114 DLBCL patients, but both methods were found to be predictive.3 However, a subgroup analysis of this study showed that the prognostic capabilities of ΔSUVmax were lower in low-risk patients or in patients treated by dose-dense–dose-intense immunochemotherapy (rituximab, cyclophosphamide, hydroxydoxorubicine, vincristine, and prednisone [R-CHOP]-14 or doxorubicine, cyclophosphamide, vindesine, bleomycine, and prednisone [R-ACVBP]), which could induce an inflammatory reaction. The residual SUVmax may be too high due to an inflammatory reaction and would require a higher ΔSUVmax. Moreover, the SUVmax in the baseline PET can be so low in some low-risk patients that the resulting ΔSUVmax value was lower than the cutoff.
The study by Schöder et al reported in this issue of Blood has several important features. First, this is a prospective study performed in a relatively good-risk DLBCL population (63% of patients with a low/low-intermediate international prognositc index [IPI]). The study included a thorough description of the quality assessment/control among PET centers participating in the study and the blinded independent central review of PET scans at the core laboratory of the study. Furthermore, the study conducted a precise comparison of the results obtained with visual DS and with ΔSUVmax. The authors strictly followed the recommendations and scored a PET positive (DS4) if visually the residual uptake was “moderately” increased compared with the liver.4 This decreases the number of false positive studies that can occur when DS4 is not reported visually but when the residual SUVmax is just over the liver SUVmax. Second, the difference in predictive value of PET2 depends on the PET2 reading criteria used and the heterogeneity of DLBCL patients included in the study, which may vary with different disease risk, treatment intensity, and the use (or not) of PET-guided therapy. Three trials explored the predictive value of i-PET in DLBCL: the Swiss SAKK 38/07 trial,5 the French LNH 07-3B,6 and the PETAL trial.7 In SAKK 38/07, which included 136 low-risk patients, most with a low or low-intermediate IPI (71%) treated with a non–PET-guided R-CHOP-14, visual assessment with DS was predictive on event-free survival (EFS), but not on OS, whereas the opposite was found by ΔSUVmax. Both the low-risk composition and the dose-dense R-CHOP-14 regimen are possible explanations for the better performance of the visual assessment. Both the French and the German PET2–guided prospective trials showed the superiority of ΔSUVmax over visual analysis. LNH 07-3B included 220 patients under the age of 60, with 97% with an age-adjusted IPI of 2 or 3 who were treated with R-CHOP-14 or R-ACVBP. PETAL included 596 DLBCL patients, 61% with low/low-intermediate IPI, who were treated with R-CHOP-14. PETAL provided for a 21-day interval between cycles 2 and 3 to avoid false positive i-PET findings due to inflammatory reactions. Of 270 patients with a DS ≥ 3, 214 (79%) had a ΔSUVmax ≥ 66%. The hazard ratios for a positive i-PET were higher for ΔSUVmax than for DS in any time-to-event end point: 3.13 in EFS as compared with 1.38; 3.48 in OS vs 1.91; 3.06 in PFS compared with 1.34; and 3.04 vs 1.46 in time to progression. The results of Schöder et al confirmed these results. The lack of significance for the ΔSUVmax of PET2 and PFS is probably due to the small number of events, especially in the R-DA-EPOCH arm of the study.
The lack of predictive accuracy of the DS reading in the EoT PET was “surprising” even for the authors of the present article and was discrepant with the published literature on the predictive value for long-term disease control of EoT PET.8,9 One explanation could be selection bias, as the authors themselves acknowledge, with better prognosis patients participating in the imaging substudy compared with those enrolled in just the parent study. This explanation is supported by a superior 2-year PFS of the imaging cohort compared with the parent study: 81.5% vs 78.9% (DAEPOCH-R) or 75.5% (R-CHOP). However, 3 other reasons could explain these results: (1) the low number of events in patients showing a positive EoT PET, whatever the method used, qualitative or semi-quantitative, in a relatively small cohort of patients (27/141 or 16/147 having a positive EoT PET); (2) the withdrawal from the analysis of patients progressing before the sixth cycle of chemotherapy; and (3) a relatively large number of false positive results reported (but not excluded by the analysis).
Finally, as pointed out by the authors, it is mandatory to identify, in the larger clinical context of DLBCL, predictive factors beyond i-PET, because of a relatively large number of events occurring in PET2-negative patients. In this regard, the combination of ΔSUVmax and baseline metabolic tumor volume in an ancillary study of the PETAL studies supports the need for additional predictive tools10 (see figure).
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal