In this issue of Blood, Goyal and colleagues present the second consensus guidelines for the clinical diagnosis and treatment of patients with Erdheim-Chester disease (ECD), in the era of targeted therapies.1
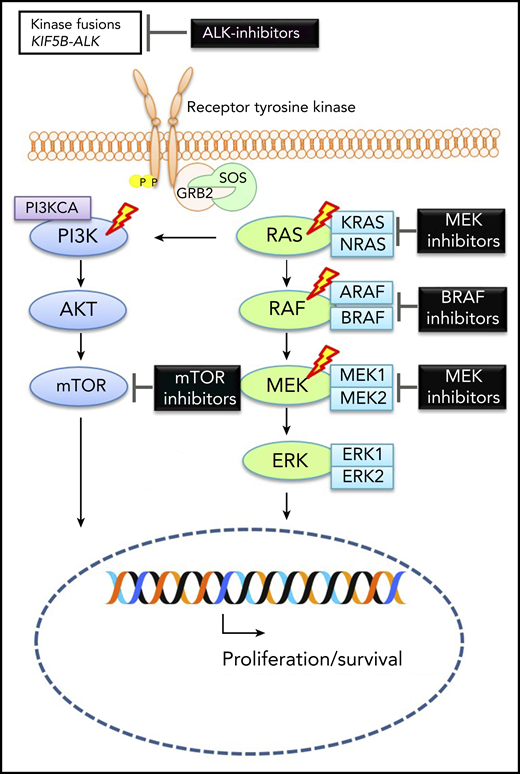
Molecular alterations in ECD. Graphic depicting MAP-kinase pathway signaling in ECD with therapeutic targets. The lightning bolts indicate most common genes that are altered in ECD. See Figure 1A in the article by Goyal et al that begins on page 1929.
Molecular alterations in ECD. Graphic depicting MAP-kinase pathway signaling in ECD with therapeutic targets. The lightning bolts indicate most common genes that are altered in ECD. See Figure 1A in the article by Goyal et al that begins on page 1929.
ECD is a rare non-Langerhans cell histiocytosis (non-LCH) with a wide spectrum of clinical manifestations, ranging from the mildest asymptomatic to life-threatening disseminated forms. It affects mainly adults and is characterized by the accumulation of CD1a-negative histiocytes in bone, kidneys, retroperitoneum, heart, lungs, skin, orbits, and brain.2 ECD is now considered an inflammatory myeloid neoplasm.1 The first consensus paper on ECD was published in 2014,2 but since then, many reports have shown additional recurrent somatic mutations beyond the BRAF-V600E oncogene and efficacy of BRAF and MEK inhibitors in ECD cases with mutations in the MAPK pathway. In 2019, members of the ECD Global Alliance reached another consensus on the molecular diagnosis, radiographic evaluations, and treatment recommendations for patients with ECD, based on expert opinion and recent literature.
The authors describe the molecular pathogenesis of ECD beyond the BRAF-V600E mutation (see figure). Almost 50% of ECD cases are driven by activating mutations in the BRAF-V600E and another 25% by activating mutations in MAP2K1.3 Mutations in ARAF, NRAS, KRAS, and PIK3CA are more common in ECD than LCH, as are translocations leading to fusions of BRAF, ALK, and NTRK1.3 However, ∼10% of ECD cases have an unexplained pathogenesis, and it will be useful to dissect their molecular signatures. More functional genomic characterization of the ALK and NTRK1 fusion genes and assessment of epigenetic mechanisms in the pathogenesis of ECD are warranted.
A biopsy of lesional tissue is highly recommended in patients with ECD, even in the presence of the classical bilateral long bone involvement with cardiovascular, lung, and retroperitoneal disease. The presence of Touton giant cells with strong positivity for factor XIIIa is typical of ECD.1 The different methods of molecular assessment of tissue for alterations in MAPK-ERK and other pathways were presented by the authors. In ECD cases where tissue samples are insufficient for molecular analysis, cell-free DNA should be assessed.4 Furthermore, all patients at diagnosis should be evaluated by total body fluorodeoxyglucose–positron emission tomography–computed tomography, in addition to brain and heart magnetic resonance imaging. Patients with unexplained cytopenia, leukocytosis, or monocytosis should undergo a bone marrow biopsy, due to the high incidence of concurrent myeloid neoplasms in patients with ECD.1
The stratification of patients according to disease severity, organ dysfunction, and mutational status is quite helpful in treatment decisions. Asymptomatic patients or those with minimal-burden disease can be monitored closely. Systemic steroids, radiation therapy, and surgery are not recommended except for acute symptoms or edema. Patients with low-burden disease involving the bones or retroperitoneum may benefit from interleukin-1 receptor antagonist, anakinra, as first-line therapy.
BRAF inhibitors, vemurafenib or dabrafenib, have become first-line therapy for all patients with BRAF-V600E–mutated ECD who have symptomatic bony, cardiac, or neurologic disease. ECD patients without BRAF-V600E mutation should undergo targeted-capture next-generation sequencing to test for mutations in the MAPK-ERK or PI3K-AKT pathways; if these patients are symptomatic, then empiric treatment with MEK inhibitors, cobimetinib or trametinib, can be considered. For patients without access to inhibitor therapies who are very symptomatic, cladribine or interferon-α/pegylated interferon can be effective.1
Although BRAF-inhibitor therapy achieved dramatic responses in many ECD patients,5,6 there are caveats. First, the optimal duration of these drugs is unknown since the “LOVE” study showed that 75% of ECD patients who stopped vemurafenib relapsed within 6 months; however, all patients responded again when the drug was restarted.7 Another limitation is toxicity (skin rash, fatigue, arthralgia, fever), and many patients will require a dose reduction due to intolerance.5 Also, BRAF inhibitors paradoxically increase the risk of secondary cutaneous neoplasia by activating RAS signaling in BRAF–wild-type cells, which could rarely cause pancreatitis and sarcoidosis as well.1 Furthermore, although resistance to BRAF inhibitors is more common in melanoma patients, a new KRAS mutant lesion after treatment with dabrafenib has occurred in 1 BRAF-V600E–mutated ECD patient.8
MEK inhibitors have recently shown excellent responses in refractory ECD patients without BRAF-V600E mutation or those who could not tolerate BRAF inhibitors due to toxicity.9 However, these patients need to be carefully monitored for ocular (retinal vein occlusion) and cardiac toxicity due to MEK inhibition.9 In addition, no data exist regarding discontinuation of MEK-inhibitor therapy and frequency of subsequent relapses. Combination of BRAF and MEK inhibitors may not be needed in ECD patients, unlike melanoma, because ECD is quite sensitive to kinase inhibitor monotherapy. There are also concerns about additive toxicities such as cardiac failure and fatigue when using both inhibitors.1 Nevertheless, patients with suboptimal response to BRAF-inhibitor monotherapy or severe cutaneous toxicity might benefit from combination therapy. In all patients with ECD, a careful discussion of the risks and benefits of these drugs is warranted. Furthermore, the cost of these inhibitors can be prohibitive in many countries, but a few alternative therapeutic options are shown in Table 1 in the article by Goyal et al.
ECD is quite rare in children. It is not always easy to distinguish between pediatric systemic juvenile xanthogranuloma and pediatric ECD; it remains to be determined whether the treatment recommendations are applicable to this age group. A multicenter pediatric ECD case series would be helpful.
Future studies are needed to evaluate the role of new targeted drugs beyond BRAF and MEK inhibitors in ECD. An ALK inhibitor, crizotinib, has been successfully used in an ECD patient harboring a KIF5BALK fusion. Inhibitors of ERK, NTRK1, and CSF1R mutations are other potential agents to be studied.10 Questions remain regarding the best biomarker to assess treatment response, optimal dosing, and treatment duration of BRAF or MEK inhibitors in ECD patients, and these can only be answered through international collaboration in well-designed clinical trials.
Conflict-of-interest disclosure: O.A. declares no competing financial interests.