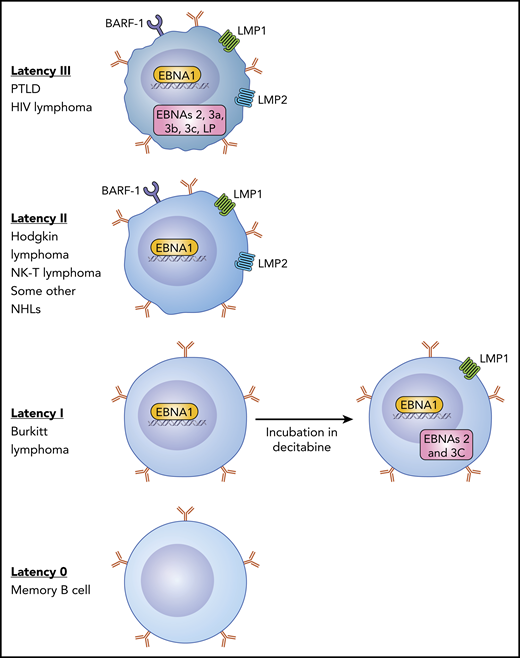
In this issue of Blood, Dalton et al1 show that epigenetic therapy with decitabine can upregulate immunogenic Epstein-Barr virus (EBV) antigens on Burkitt lymphoma (BL) that normally only express the less immunogenic antigen EBV nuclear antigen-1 (EBNA-1), rendering them sensitive to EBV-specific cells. The authors hypothesized that inducing expression of the more immunogenic latent viral antigens expressed in EBV type II and III latency tumors, such as posttransplant lymphoproliferative disease (PTLD), on EBV I latency tumors like BL, could improve the activity of virus-directed immunotherapies against these tumors.
There are 4 EBV latency states in B cells. Type III latency is the most immunogenic with expression of all of the latency-associated proteins that induce B-cell transformation and is seen in lymphomas that arise in individuals with profound immunosuppression such as PTLD. Type II latency tumors that include some cases of Hodgkin lymphoma and some NHLs express EBNA1, LMP1, and LMP2 and have intermediate immunogenicity. BL expresses type I latency with only EBNA1 expressed and is poorly susceptible to an EBV-CTL response. In type 0 latency, seen in normal memory B cells, no viral genes are expressed. Dalton et al show that incubation of type I latency lymphoma cells with decitabine can result in upregulation of the LMP1 and EBNA2 genes, making these cells more susceptible to EBV-CTLs. NK-T, natural killer T cell.
There are 4 EBV latency states in B cells. Type III latency is the most immunogenic with expression of all of the latency-associated proteins that induce B-cell transformation and is seen in lymphomas that arise in individuals with profound immunosuppression such as PTLD. Type II latency tumors that include some cases of Hodgkin lymphoma and some NHLs express EBNA1, LMP1, and LMP2 and have intermediate immunogenicity. BL expresses type I latency with only EBNA1 expressed and is poorly susceptible to an EBV-CTL response. In type 0 latency, seen in normal memory B cells, no viral genes are expressed. Dalton et al show that incubation of type I latency lymphoma cells with decitabine can result in upregulation of the LMP1 and EBNA2 genes, making these cells more susceptible to EBV-CTLs. NK-T, natural killer T cell.
EBV is a ubiquitous human herpes virus with a marked B-lymphotropism, and by adulthood, >95% of the population have had a primary infection, where B-lymphocytes are infected and express viral latency-associated proteins after initial lytic infection in the oropharynx with viral shedding. During primary infection and recovery, the transition from a newly infected B cell to infected memory B cell involves 3 stages of latency, and each latency type is associated with specific B-cell malignancies (see figure). Normal individuals have lifelong EBV persistence with “true latency” in a subset of memory B cells that have no expression of viral genes (latency 0).2 Seropositive individuals will have periodic reactivations in B cells with expression of lytic and latent EBV antigens, which are tightly controlled by a strong viral antigen-specific T-cell response.2
Nine latency proteins, including nuclear (EBNAs), membrane proteins (LMPs), and the secreted BARF1 gene product, are expressed in PTLD, which is a type III latency disease that arises in immunocompromised patients.2 These antigens are highly immunogenic and so are controlled in immunocompetent individuals by an EBV-specific cytotoxic T-cell (EBV-CTL) response. However, in individuals who are immunosuppressed, such as after transplant, B cells expressing these antigens can outgrow, resulting in EBV PTLD. Several groups have shown that reactivation and expansion of either transplant donor or third-party T cells specific for EBV latency antigens from healthy seropositive donors can effectively prevent and treat EBV+ lymphomas after allogeneic hematopoietic stem cell transplant (HSCT) or solid organ transplant.3-5
By contrast, type II latency tumors, such as Hodgkin lymphoma, some types of non-Hodgkin lymphoma (NHL), and nasopharyngeal carcinoma (NPC), arise in immunocompetent persons and express a more limited array of less immunogenic antigens: LMP1, LMP2, EBNA1, and BARF1. Based on the success of EBV-CTL therapy in treating immunogenic type III latency tumors, clinical studies tested the efficacy of these therapies for type II latency EBV-associated malignancies. Therapy with autologous EBV-CTLs has elicited tumor responses in >50% of patients with relapsed lymphoma and 20% to 30% of patients with NPC.6,7 Of note, responders developed immune responses to other tumor-associated antigens due to epitope spreading.6
Type I latency tumors, like BL, only express EBNA-1 and EBV-encoded small RNAs. Although EBNA1-specific T cells have been used successfully to treat patients with PTLD following HSCT,8 EBV-specific CTLs have not yet been evaluated in BL. To develop a treatment of type I latency EBV tumors, such as BL, Dalton et al explored combining epigenetic drugs that can increase the expression of tumor antigens to engage cognate T-cell receptors with adoptive T-cell therapy. In a high-throughput screen, they identified the epigenetic modulators decitabine and 5-azacytidine as potent inducers of immunogenic latent EBV antigens in latency I EBV+ tumors. The authors then performed in vitro studies to show that decitabine was more effective in upregulating LMP1, EBNA 2, and 3C and rendering BL tumors susceptible to T-cell–mediated lysis with EBV-CTLs. Furthermore, decitabine followed by EBV-CTLs results in T-cell homing to tumors and inhibition of tumor growth in BL xenograft models, suggesting a novel therapeutic approach to sensitize EBV+ lymphomas to immunotherapy. Of note, the effect of decitabine persisted for days to weeks after incubation, suggesting that the therapies could be sequenced in clinical testing using decitabine as “preconditioning” to upregulate latent antigen expression and sensitize malignant cells to T-cell–mediated lysis. EBV methylation analysis showed that decitabine produces global hypomethylation across key latency promoters.
Therapeutic strategies targeting EBV have previously been evaluated with histone deacetylase inhibitors, such as arginine butyrate and nanatinostat, which induce lytic viral replication in EBV-associated malignancies to sensitize them to antivirals, such as ganciclovir.9,10 Lytic antigens can also be targets for CTL responses, and Dalton et al noted that decitabine also upregulated some lytic antigens, such as BZLF1, although to a lesser degree than 5-azacytidine and with a more transient effect than observed with the latent antigens.
One concern with the strategy proposed here is that, following decitabine, some BL cells did not express latency II or III antigens, suggesting there may be selection for the clones with latency I phenotype after EBV-CTL infusion. Therefore, further evaluation of combinations that might induce expression of latency or lytic antigens in a higher percentage of cells is needed. It will also be of interest to learn if decitabine can induce expression of other nonviral tumor-associated antigens. Regardless, this combination merits clinical evaluation, and trials should illuminate whether increased expression of EBV latent and lytic antigens in malignant cells makes them more susceptible to EBV-specific T cells.
Conflict-of-interest disclosure: H.E.H. is a cofounder with equity in Allovir and Marker Therapeutics, has served on advisory boards for Tessa Therapeutics, Kiadis, Gilead Biosciences, Novartis, and PACT Pharma, and has received research support from Tessa Therapeutics and Cell Medica.