In this issue of Blood, Vercellino and coworkers1 clearly demonstrate that pretreatment total metabolic tumor volume (TMTV) predicts progression-free survival (PFS) and overall survival (OS) in patients with diffuse large B-cell lymphoma (DLBCL), even among those responding to treatment.
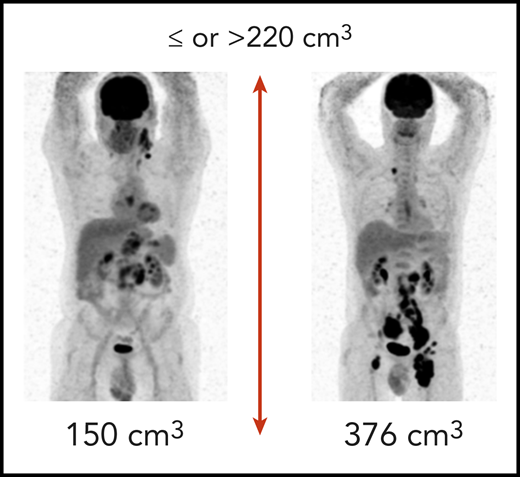
Examples of patients from the REMARC trial with low and high total metabolic tumor volume. (Courtesy of M. Meignan and C. Thieblemont.)
Examples of patients from the REMARC trial with low and high total metabolic tumor volume. (Courtesy of M. Meignan and C. Thieblemont.)
Whereas ∼60% of patients with DLBCL are cured with standard chemoimmunotherapy, 20% are refractory to initial treatment, and another 20% will relapse and potentially die of the disease. In the 27 years since publication of the International Prognostic Index (IPI),2 numerous modifications have been proposed, including the Revised IPI, the age-adjusted IPI, and the National Comprehensive Cancer Network IPI. All of these systems used standard clinical and laboratory factors to predict outcome; however, none direct treatment strategies but serve more to help stratify patients for clinical trials. Other clinical factors, such as tumor size, have also correlated with outcome; however, the definition of “bulky disease” has varied among studies from 5 cm to 10 cm.3
TMTV takes the next step by combining size (volume) with metabolic activity as measured by 2′-fluorodeoxyglucose uptake. This imaging technique performed pretreatment is predictive of outcome not only in DLBCL but also in other histologies, including Hodgkin lymphoma, follicular lymphoma, and primary mediastinal lymphoma.4 The current data were derived from the REMARC trial, which included “elderly” patients who were treated with R-CHOP (conventional rituximab, cyclophosphamide, adriamycin, vincristine, and prednisone), with responders randomized to lenalidomide maintenance or placebo. The results clearly reinforce that simply responding to therapy DLBCL is necessary, but insufficient for prolonged outcome. In a subset of patients, pretreatment TMTV was calculated with a threshold of 220 cm3 selected and validated as the optimal cutoff for PFS and OS (see figure). All patients experienced a complete (75%) or partial (25%) response. Nonetheless, the 159 patients with a high pretreatment TMTV (>220 cm3) had a significantly shorter PFS and OS than the 142 patients with a lower TMTV. For high vs low TMTV, the 4-year PFS was 56% vs 82% and 4-year OS was 74% vs 92%, respectively. These differences existed regardless of treatment arm. Of the numerous clinical and laboratory factors examined, only performance status (PS) complemented TMTV. They developed a prognostic score using TMTV and PS to identify ultra-high-risk patients who might be candidates for alternate treatment approaches; with no factors, the 4-year PFS was 82% compared with 63% for those with either TMTV >220 or Eastern Cooperative Oncology Group ≥2, and only 41% for patients with both adverse risk factors. Similarly, 4-year OS was 94% for those with no risk factors, 79% for patients with 1 factor, and 59% for patients with both risk factors. One piece of missing data is the outcome of the complete responses compared with the partial responses. Nonetheless, with a highly reproducible imaging study and a simple clinical estimate, the authors were able to predict patients likely to benefit most from therapy before receiving the first dose.
However, a galaxy remains between where we are and where we need to be. It is 1 thing to identify those patients with a dismal outcome, and quite another to design approaches to modify the future. Recognition of differences in cell of origin in DLBCL was an initial glance into tumor heterogeneity.5 Unfortunately, modifying treatments on the basis of that information has been futile to date.6 More recently, at least 2 groups have distinguished DLBCL into clinically relevant molecular subtypes pretreatment. Chapuy et al7 identified 5 subsets, including a group of low-risk activated B-cell (ABC)-DLBCLs of extrafollicular/marginal zone origin; 2 subsets of germinal center (GCB)-DLBCLs with different outcomes; and an ABC/GCB-independent group with inactivation of TP53, CDKN2A loss, and associated genomic instability, with 10-year PFS ranging from 40% to 70%. Schmitz et al8 identified 4 subsets with different recurrent genetic and epigenetic alterations, including MYD88L265P, CD79b, BCL6 fusions and NOTCH2 mutations, and EZH2 mutations, with 10-year PFS ranging from 0% to 10% to 60%. What is significant about both of these observations is not only do they appear to add to the IPI but also, more importantly, that some of the alterations are targetable.
Therefore, if pretreatment TMTV groups can be separated by a difference in survival of 20%, and molecular subsetting discriminates those with a survival ranging between 0% and 10% and 70%, why do we treat all patients the same and despair when the unfavorable groups relapse? Studies such as that by Vercellino et al provide encouragement that a risk-adapted strategy is feasible for patients with DLBCL. TMTV plus PS is a relatively simple means of identifying the lowest-risk patients. Within the unfavorable group, genetic and molecular subtyping can not only provide further separation by risk but also suggest potential targets, with some specific agents already available (eg, tazemetostat, Bruton tyrosine kinase inhibitors, venetoclax). The goal of treatment should be anticipatory: patients at low risk may be treated with conventional approaches, unless an appropriate clinical trial is available. Standard drugs should be eschewed for those unlikely to experience a favorable long-term outcome, who should instead be entered onto upfront studies exploring the efficacy of novel regimens directed at specific targets. Both groups should be monitored during treatment, with treatment modified based on the appearance of new mutations.
Therefore, to quote the sage, Yogi Berra, “The future ain’t what it used to be.” If we are going to change that future for patients with DLBCL, we will have to do it before they are ever treated.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal