TO THE EDITOR:
Involved-field radiotherapy (RT) is a well-accepted initial treatment option in patients with localized stage I extranodal marginal zone lymphoma (EMZL), resulting in excellent clinical outcomes in majority of anatomic locations.1-6 RT results in complete response (CR) in >92% of stage I patients, with a 5-year overall survival (OS) and cause-specific survival of 94% and 99%, respectively.1,7 Disease relapses are observed in 8% to 19% of patients, with the majority (80%) occurring within the first 5 years of RT, usually outside of the radiation field.1,3,4,8 Relapses are more commonly observed in distant extranodal sites but may involve local lymph nodes, spleen, and bone marrow (BM).9 Thyroid and gastric presentations are associated with a significantly lower risk of distant relapses1 in comparison with other EMZL presentations.10
For decades, BM biopsy has been a cornerstone of lymphoma staging to prove localized disease.11,12 BM biopsy is used to verify localized presentation in patients with negative imaging studies, identifying patients who can be cured with radiotherapy and spared systemic effects of immunochemotherapy. Recently, several studies evaluating the role of staging positron emission tomography/computed tomography (PET/CT) scans have challenged the need for BM biopsy in other types of lymphoma.13-16 Nevertheless, insufficient data are presently available on the sensitivity and specificity of PET/CT scans for detection of extranodal and BM involvement in EMZL; therefore, staging BM biopsy remains endorsed by European and American cancer care guidelines.17,18
Occasionally, BM biopsy is not performed in routine practice for reasons including comorbidities, patient refusal, or physician decision. It is presently unknown whether treatment decisions based on clinically/imaging-based stage I EMZL would affect lymphoma-specific survival in patients treated with RT. Therefore, we analyzed the effects of BM biopsy status (negative vs not done) on disease relapse/progression and survival in patients with EMZL presenting with clinically/imaging-based stage I disease and treated with RT.
From January 1995 to January 2019, we identified 188 patients with stage I EMZL treated with frontline RT with a curative intent in the University of Miami Health System. Patients with stage I gastric EMZL who failed Helicobacter pylori therapy and were subsequently treated with RT were included in this analysis. The institutional review board approved this study, which followed the tenets of the Declaration of Helsinki.
All patients had biopsy-proven EMZL reviewed and reassessed for this study by expert hematopathologists (J.R.C. and F.V.) following World Health Organization (WHO) classification criteria.19 The BM examination included flow cytometry analysis in all patients using a standard screening panel to detect the presence of monoclonal B cells or B cells with immunophenotypic aberrancies. Medical records were reviewed to obtain pertinent patient information.
Staging evaluation was not standardized during the study interval, but included a complete physical examination, hematological and chemical survey, and CT scans of the chest, abdomen, and pelvis in all patients. Lactate dehydrogenase levels and a CT scan of the neck were performed in most patients. Endoscopies and orbit or other magnetic resonance imaging were performed if clinically indicated. The decision to perform a staging BM biopsy was left to the discretion of the treating oncologist. PET/CT scanning was not routinely performed at diagnosis (N = 26). Relapse/progression was subclassified based on disease location: inside, outside, or inside and outside of the radiation field (RF).
Demographic and clinical characteristics were summarized using descriptive statistics. Progression-free survival (PFS) was defined as the time from diagnosis to progression, relapse, or death, whichever occurred first. OS was defined as the time from diagnosis to death. Event-free patients were censored at the date of last follow-up. PFS and OS were estimated using the Kaplan-Meier method and compared using the log-rank test. The competing risk method and the Gray test were used in analyses of cumulative incidence rates of type of relapse/progression and lymphoma-specific death.20,21 Multivariable analyses were conducted using the Cox regression or the Fine-Gray subdistribution regression. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Among the 188 patients included in this study (supplemental Table 1, available on the Blood Web site), 104 were ≤60 years (55.3%) and 135 <70 years (71.8%), with 117 females (62.2%) and 98 non-Hispanic whites (52.1%). A total of 118 ocular adnexa presentations (62.8%) and 19 gastric presentations (10.1%) were the most common EMZL locations. Staging BM biopsy was negative in 148 patients (78.7%) and not performed in 40 patients (21.3%). Patients without BM biopsy at diagnosis were mainly older than 60 years: 26 (65%) compared with 58 (39.2%) in patients with negative BM biopsy (P = .004) (supplemental Table 1). Radiation doses varied (between 4 and 46 Gy; median, 36 Gy), but most patients (176; 93.6%) received ≥30 Gy. A total of 183 patients (97.3%) achieved CR following RT with 2 achieving partial response (1.1%), 2 stable disease (1.1%), and 1 demonstrating disease progression (0.5%). Among 176 patients receiving ≥30 Gy RT, 173 achieved CR (98.2%).
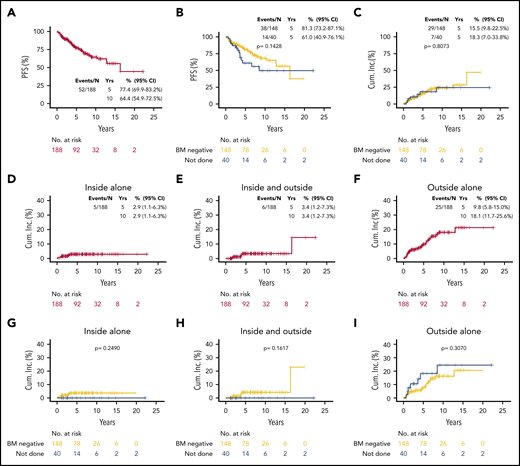
With a median follow-up of 6.2 years (0.3-22.3 years) for all the patients, the 10-year PFS was 64.4% (95% confidence interval [CI], 54.9% to 72.5%) (Figure 1A). Seven patients (3.7%) experienced higher-grade transformation to diffuse large B-cell lymphoma (6 BM biopsy–negative at diagnosis and 1 not done). No difference in PFS was observed by BM biopsy status (negative vs not done; P=.1428; Figure 1B) or by lymphoma presentation location (supplemental Figure 1A). Patients treated with ≥30 Gy had statistically significantly (P < .0001) longer PFS compared with patients treated with <30 Gy (supplemental Figure 1B). The positive statistically significant effect of >30 Gy RT, when adjusted for other prognostic factors, was confirmed in analyses of PFS, cumulative incidence of progression/relapse censoring nonlymphoma deaths, and progression/relapse accounting for nonlymphoma deaths as a competing risk (supplemental Table 2). RT dose in EMZL remains a controversial area and currently recommended doses22 are based on 2 randomized studies that mainly included patients with other lymphoma subtypes with a primary end point of local control and not PFS and OS.23,24 Although no difference was found in PFS, these studies were not powered to find such a difference. Therefore, the recommendations are mainly based on physician experience without solid data to support recommended doses in patients with stage I EMZL treated with curative intent. Although in our cohort only a few patients were treated with lower doses of RT, representing a limitation of this study, the statistically significant effect of >30 Gy RT was confirmed in various multivariable analyses.
Outcomes of EMZL patients. PFS (A-B), and cumulative incidence of each type of progression/relapse relative to RF (inside alone, inside and outside, outside alone) overall (D-F) and by BM biopsy status (C,G-I).
Outcomes of EMZL patients. PFS (A-B), and cumulative incidence of each type of progression/relapse relative to RF (inside alone, inside and outside, outside alone) overall (D-F) and by BM biopsy status (C,G-I).
There were 52 progression events, including 16 nonlymphoma deaths and 36 progression/relapse events: 5 inside RF (4 relapses/1 progression), 6 inside and outside (5 relapses/1 progression), and 25 outside alone (24 relapses/1 progression). There was no higher cumulative incidence of relapse/progression (P = .807, all patients [Figure 1C]; and P = .982, in patients achieving CR [supplemental Figure 2B]) in patients without vs negative staging BM biopsy. Taking into account nonlymphoma death as a competing risk, the 5-year incidence of lymphoma relapse/progression was 16.1% (95% CI, 10.8% to 22.3%) overall (supplemental Figure 2A), 2.9% (95% CI, 1.1% to 6.3%) inside RF (Figure 1D), 3.4% (95% CI, 1.2-3.7) inside and outside RF (Figure 1E), and 9.8% (5.8% to 15.0%) outside RF (Figure 1F). Importantly, there was no higher incidence of each type of relapse/progression relative to RF (inside P = .2490, inside and outside P = .1617, and outside alone P = .3070) in patients without vs negative staging BM biopsy (Figure 1G-I).
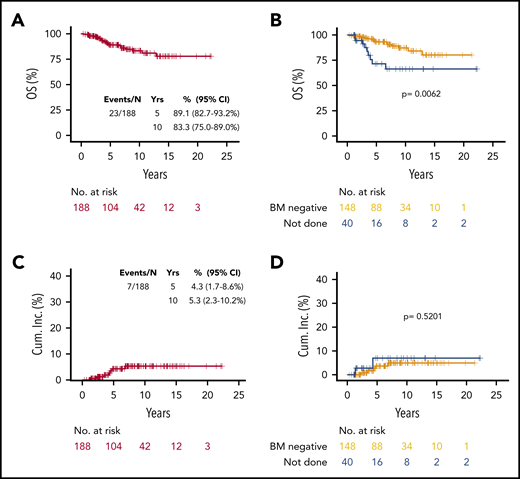
There were 23 deaths, 7 attributed to lymphoma. The 10-year OS was 83.3% (95% CI, 75.0% to 89.0%) (Figure 2A). Patients without BM biopsy had shorter OS (P = .0062) (Figure 2B), and there was no difference in OS by EMZL location (supplemental Figure 1C). However, when lymphoma-specific death was analyzed (nonlymphoma death as competing risk), the estimated 10-year cumulative incidence of lymphoma-specific death was 5.3% (2.3% to 10.2%) (Figure 2C) and there was no statistically significant effect of BM biopsy (P = .5201) (Figure 2D). The cumulative 10-year incidence of nonlymphoma-specific death (lymphoma death as competing risk) was 11.4% (95% CI, 6.3% to 18.2%) (supplemental Figure 3A). There was a statistically significant difference in nonlymphoma specific death (lymphoma death as competing risk) by BM biopsy status (P = .0107) (supplemental Figure 3B), with patients without staging BM biopsy having higher death incidence compared with patients with negative BM (10-year rate, 26.7% vs 7.9%, respectively). The negative effects of BM biopsy not done (hazard ratio [HR] = 2.85; P = .034) and age ≥70 years (HR = 3.76; P = .012) but not of RT dose ≥30 Gy (HR = 0.38; P = .132) on nonlymphoma-specific death were confirmed in a multivariable model (supplemental Table 3).
OS and incidence of lymphoma-specific death in EMZL patients. OS in all patients (A), and by BM biopsy status (B), and cumulative incidence of lymphoma-specific death in all patients (C) and by BM biopsy status (D).
OS and incidence of lymphoma-specific death in EMZL patients. OS in all patients (A), and by BM biopsy status (B), and cumulative incidence of lymphoma-specific death in all patients (C) and by BM biopsy status (D).
Herein, we show that patients with stage I EMZL exhibit excellent response, PFS, and OS following frontline RT, as previously reported.4-6 Furthermore, we observed similar lymphoma-specific outcomes in clinically/imaging-based and BM biopsy–staged stage I EMZL patients. In patients with and without diagnostic BM biopsy, most relapses occurred outside of the RF with similar cumulative incidence of each type of relapse. These findings suggest that diagnostic BM biopsy may not be required and does not affect lymphoma-related outcomes in patients with clinically/imaging-based stage I EMZL treated with RT. However, our study has limitations inherent to all retrospective studies, including nonuniform indications for nonperformance of BM biopsy, differences in the radiation doses and methodology over the years, limited number of patients treated with lower doses of RT, and variability in follow-up duration and testing done during the follow-up. Further studies are needed to confirm these observations before recommendations on omitting BM biopsy in patients with stage I EMZL treated with frontline RT can be made.
Presented at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7-10 December 2019 (Abstract 2829).
The online version of this article contains a data supplement.
Acknowledgments
J.P.A. is a K12 Scholar supported by the National Cancer Institute, National Institutes of Health under award number K12CA226330. I.S.L. was supported by grant 1R01CA233945 from the National Institutes of Health, National Cancer Institute; Florida Health Bankhead-Coley Cancer Research award AWD-005151; the Dwoskin, Recio, and Anthony Rizzo Families Foundations; and the Jaime Erin Follicular Lymphoma Research Consortium.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.P.A. collected the data, conceptualized and designed the study, analyzed the data, and wrote the manuscript; D.I., S.G.I., and J.J.M. collected the data; W.Z. and I.M.R. analyzed the data and wrote the manuscript; J.R.C. and F.V. performed review of diagnostic biopsies and confirmed diagnoses; A.M.M. was involved in the treatment of these patients; I.S.L. conceptualized and designed the study, was involved in the treatment of these patients, analyzed the data, and wrote the manuscript; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: J.P.A. has received honoraria from Targeted Oncology, OncLive, and Oncinfo, and has an immediate family member who has served on advisory boards for Puma Biotechnology, Inovio Pharmaceuticals, Agios Pharmaceuticals, Forma Therapeutics, and Foundation Medicine. I.S.L. has served on advisory boards for Seattle Genetics, Janssen Scientific, and Verastem. The remaining authors declare no competing financial interests.
The current affiliation for F.V. is MD Anderson Cancer Center, Houston, TX.
Correspondence: Izidore S. Lossos, Division of Hematology, Department of Medicine, Miller School of Medicine, University of Miami, 1475 NW 12th Ave (D8-4), Miami, FL 33136; e-mail: ilossos@med.miami.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal