Introduction: Mixed donor chimerism (MDC) occurs in nearly 35% of patients with sickle cell disease (SCD) post allogeneic hematopoietic cell transplant (alloHCT). Donor myeloid engraftment of >20% is considered necessary to support disease resolution. However, in a disease known for its clinical variability, we hypothesize that patients (pts) with a severe pre-HCT phenotype may have abnormal RBC rheology at a level of chimerism and %HbS believed to be consistent with a cure. RBC rheology is markedly abnormal in SCD; these abnormalities are associated with SCD related clinical complications. Even fully oxygenated, sickle RBC are less deformable than those of HbAA or HbAS individuals; upon deoxygenation, deformability further declines. Sickle RBC morphology reflects the tendency to polymerize, with characteristic sickle forms. Sickle RBC are more adherent to the endothelial cell lining of the vasculature than HbAA or HbAS RBCs. The goal of any cell-based therapy of curative intent should be normalize the red cell rheology. To determine if the rheology of blood from SCD pts post-HCT with varying degrees of donor chimerism fall into the HbSS range of values, we propose to functionally assess the peripheral blood from a series of 6 post alloHCT SCD patients using a battery of rheological tests measuring deformability, sickling, adherence, percent dense cells (%DRBC), and RBC morphology.
Methods: Peripheral blood samples (EDTA) from 6 SCD pts post alloHCT with a matched sibling donor were collected and immediately analyzed using oxygenscan (Lorrca), artificial microvascular network (AMVN), microfluidic image acquisition, and an ADVIA hematology analyzer. The Lorrca with oxygenscan measures RBC deformability (elongation index EI), under a range of pO2 (150-0 mmHg). EImax is the deformability of the oxygenated sickle RBC; EImin is the deformability of deoxygenated RBCs. The point of sickling (PoS) is the pO2 at which RBC deformability rapidly declines. RBC adhesiveness is measured by the difference in rate of perfusion of RBC through the AVMN coated with adhesive proteins (adherent AVMN) and a non-adherent, uncoated AVMN. %DRBC was measured by an ADVIA hematology analyzer. Microscopic RBC images were acquired and classified to determine the fraction of sickled RBCs in well-oxygenated samples. Additional clinical data including Hb profile, donor chimerism, and symptoms were obtained via chart review. Reference ranges were generated as described above using n=45 HbSS samples ages 2-21, on hydroxyurea, chronic transfusion, or untreated; n=14 HbAS, and n=43 HbAA.
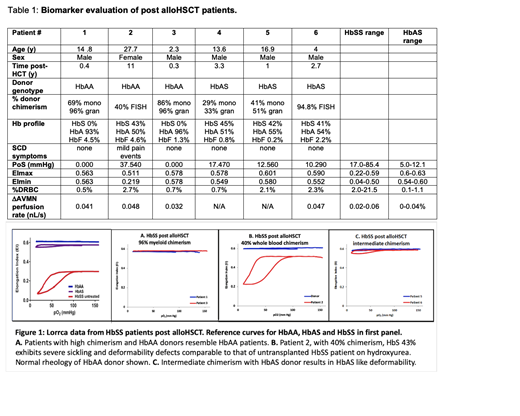
Results: In Figure 1, we show the measurements obtained by the Lorrca with oxygenscan on 5 pts post-alloHCT with a range of donor chimerism, as well as typical values for HbAA, HbAS, and HbSS patients. Patient 1 and 3 exhibit normal rheology; both were transplanted with a HbAA donor, with high chimerism and no detectable HbS. Patient 2 has 40% whole blood chimerism, well above the 20% threshold described with myeloid chimerism, and a HbS less than 50%, yet their plot resembles that of an untransplanted HbSS pt; the donor to Patient 2 exhibits the expected HbAA deformability. Patients 5 and 6 exhibit rheology in the HbAS range; they have intermediate chimerism from a HbAS donor. Clinical details and biomarker panel values for all 6 pts analyzed are in Table 1, as well as ranges of values for HbSS and HbAS subjects generated in the Sheehan and Shevkoplyas labs. Patient 2 is the only subject with values in the HbSS range, and the only subject with SCD symptoms.
Conclusions: Our rheological tests identified a pt with values consistent with a HbSS pt of moderate severity or treated with hydroxyurea despite variable donor chimerism above the 20% threshold and HbS <50% thought to be sufficient for cure. The pt reported pain events beginning two years prior, indicating a clinical correlation supporting the validity of the rheological tests we propose to use to distinguish cure from persistent SCD. Current post-HCT evaluation depends on chimerism and hemoglobin profiles, and would not detect the significant functional abnormalities visible by Lorrca with oxygenscan biomarkers that indicate that Patient 2 is at risk for SCD related complications. Our results suggest that this functional analysis may help with management of post alloHCT SCD pts, and may be even more essential to assessing new gene-based therapy approaches to curing patients with SCD.
Rab:RR Mechatronics: Research Funding. van Wijk:Agios Pharmaceuticals: Consultancy, Research Funding; RR Mechatronics: Research Funding. Shevkoplyas:SSS: Research Funding; New Health Sciences: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal