Background
Sickle cell disease (SCD) is curable by transplantation and potentially by gene therapy, and is generally treated by a combination of blood transfusion and Hydroxyurea (HU). Characterizing the hematopoietic system in SCD patients is important because the long-term effect of HU treatment are not known, and because of lower than expected efficacy of transduction and transplantation in Hematopoietic Stem and Progenitor Cells (HSPCs) in recent gene therapy trials.
Previous studies have shown that the number of bone marrow (BM) CD34+ cells is elevated in SCD patients and that HU treatment is associated with decreased level of CD34+ cells in the peripheral blood (PB) and BM relative to steady state patients. However, hematopoiesis in SCD patients naive or treated with HU or transfusion remains poorly understood.
Here, we report on the characterization of the HSPC compartment in patients with SCD by prospective isolation of 49f+ long-term Hematopoietic Stem Cells (49f+LT-HSCs), Multipotent Progenitors (MPPs), Common Myeloid Progenitors (CMPs), Megakaryocyte-Erythroid Progenitors (MEPs), and Granulocyte-Monocyte Progenitors (GMPs).
Methods
After obtaining consent, PB and/or BM were collected from 69 patients with HBSS/SB0, aged 12 to 45years, and 25 healthy adult African American controls. Patients were divided into chronic transfusion therapy (n=19), HU (n=31) and naïve (n=19) groups. Frozen mono-nuclear cells were analyzed by flow cytometry on a BD LSRII using CD 49f, 90 45Ra, 123, 235a, 38, 34, 33 and lineage antibodies.
Results
FACS analysis revealed that the number PB CD34+ cells was 2.5 CD34+/uL of blood in the HU group as compared to 19 CD34+/uL in the exchange and naive groups, and 7.3 CD34+/uL in the control group (q-value <0.05 in all cases). Analysis with additional markers revealed that the decrease in circulating HSPCs in the HU group affected the entire hematopoietic system since the number of 49f+LT-HSCs, MPPs, CMPs, MEPs were all significantly lower in the HU group.
The decrease in cell number in the HU group, however, was not homogeneous. The proportion of LT-HSCs was higher in the HU and transfusion groups when compared to the naive and control groups. The HU group also had the lowest proportion of MPPs and GMPs, as well as the highest proportion of MEPs.
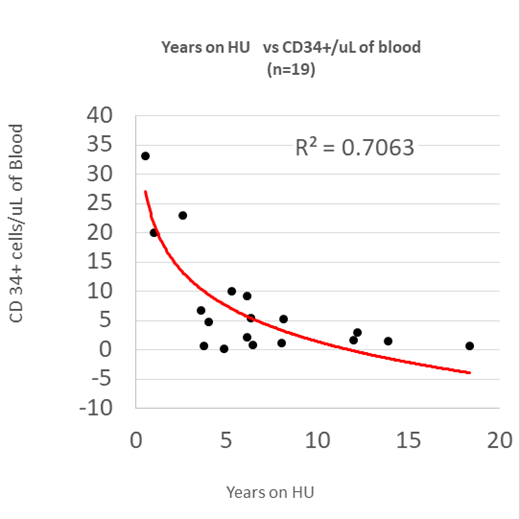
We then investigated hematopoiesis as a function of the length of HU treatment to elucidate the long-term treatment effect of this cytotoxic agent. Patients > 18 years of age that had been treated on HU for at least three years exhibited a strong statistically significant negative correlation between years on HU and CD34+/uL (R2 = 0.41), LT-HSC/uL (R2 = 0.35), MPP/uL (R2 =0.43), CMP/uL (R2 = 0.37), MEP/uL (R2 = 0.25) and GMP/uL (R2 0.39, p<0.01 in all cases). Importantly there was no correlation between WBC counts, age, HU dose, or serum erythropoietin level versus the numbers of any HSPC/uL.
Lastly, we compared the number of HSPCs in paired PB and BM samples 10 controls and 4 SCD patients. This revealed that the numbers of CD34+, HSCs, MPPs, CMPs, GMPs and MEPs in the PB and BM were well correlated (r2 in 0.6-0.8 range) suggesting that in first approximation, results obtained in the PB reflect changes in the BM rather than changes in egress of HSPCs from the BM.
Discussion
We have observed lower numbers of circulating CD34+,49f+LT- HSCs, MPPs, CMP, GMP and MEPs in individuals with HBSS on HU therapy when compared to naive, chronic transfusion and, to a lesser extent, controls. Furthermore we observed subtle differences in the proportion of various circulating stem and progenitor cells (HSPCs) suggesting that the various treatments affects hematopoiesis in complex ways.
The strong negative correlations between the length of HU treatment and the numbers of HSPCs can be explained either by decreased cell mobilization to the periphery, or by a depletion of the HSPC numbers in the BM overtime.
Most patients undergoing gene therapy trials are currently taken off HU and placed on transfusion therapy for several months to increase CD34+ cell collection and LT-HSC transduction efficiency. We observed a greater number of circulating 49f+LT-HSC/uL of blood in the transfusion group than in the HU group, but the proportion of 49f+LT-HSC relative to the number of CD34+ were similar in both groups. Functional studies may help determine whether 49f+LT-HSCs from the transfusion group are qualitatively different from of the HU group and more amenable to gene therapy.
Manwani:Novartis: Consultancy; Pfizer: Consultancy; GBT: Consultancy, Research Funding. Minniti:Doris Duke Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal