Introduction and Aim
Till today, the only curative and most widely used therapy for β-thalassemia (β-TDT) is allogeneic HSCT: the European Society for Bone Marrow Transplantation Hemoglobinopathy Registry reported over 90% overall survival in under-14 patients transplanted from an HLA identical family donor. However, with the new therapeutic scenario opened up by Gene Therapy (GT), it has become essential to identify and prioritize patient profiles for which GT could be applied. The "Pesaro Patients Risk Classification" showed the importance of risk stratification in order to achieve the best results. The Italian Scientific Society for Thalassemias and Hemoglobinopathies (SITE) closely involved in the cure and overall approach to these pathologies, decided to carry out this project of analysis and assessment to establish the possible inclusion and exclusion criteria for access to GT of patients with β-TDT and to collect the outcomes. The aim of this study is to identify which patients with β-TDT could benefit from GT, based on evidence and consensus.
Methods
The decision-making algorithm was developed within the scientific activity of SITE, which defined the feasibility of the project and selected the multidisciplinary panel of experts in hemoglobinopaties for the elaboration of clinical issues.
Published literature (Medline, PubMed, Embase, Cochrane Library) was searched in order to get solid evidences regarding best candidates to allogeneic SCT, who should not therefore undergo GT. Evaluation of literature and scientific evidences were reported and discussed through call conferences and mails communications. The final review has been performed by a pool of external reviewers who evaluated the clinical relevance, the applicability, the legibility of the document and the consistency between the recommendations and the summaries of the tests produced to test the algorithm. This step is in progress. The final version of the document will be made available on the web site of SITE (www.site-italia.org). The outcomes of the procedures will be collected in the electronic clinic database used by the Centers (i.e Webthal©) to perform a risk stratification.
Results and Conclusions
The patient selection procedure must be performed by the joint evaluation of the Center of the "Hemoglobinopathies Italian Network" together with the qualified treatment Center for HCS transplantation.
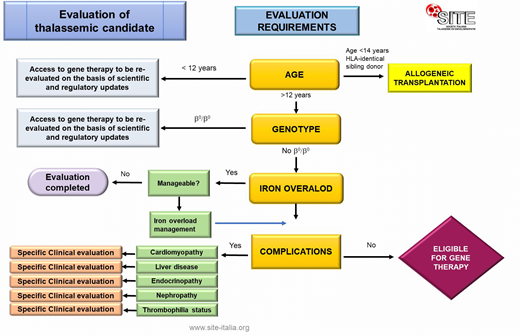
The document is proposed as a dynamic and up-datable tool. It is structured such as to allow two levels of consultation: an interactive flow chart (Figure) presenting the different clinical issues discussed with conclusive practical indications linked to chapters providing detailed information on each aspect of the subject.
Applying the appropriate cautionary considerations, patients are considered non-eligible in the case of:
exclusion criteria indicated by the Regulatory Authorities (i.e. EMA/CHMP/166977/2019);
Severe iron overload and/or organ damage (i.e. pulmonary hypertension).
Manageable iron overload is a condition of re-evaluation. Caution is mandatory in the evaluation of patients with complications or comorbidities.
The access to GT needs to be re-evaluated on the basis of scientific and regulatory updates.
Longo:Blue Bird Bio: Consultancy. Pinto:Novartis: Consultancy; Bluebird Bio.: Consultancy. Angelucci:Novatis: Honoraria, Other: Chair Steering Committee TELESTO protocol; Celgene: Honoraria, Other: Participation in DMC; BlueBirdBio: Other: Local advisory board; Jazz Pharmaceuticals: Other: Local advisory board; Roche: Other: Local advisory board; Vertex Pharmaceuticals Incorp., and CRISPR Therapeutics: Other: Participation in DMC. Cappellini:Novartis: Membership on an entity's Board of Directors or advisory committees; Vifor Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Honoraria; CRISPR Therapeutics: Membership on an entity's Board of Directors or advisory committees; Genzyme/Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees. Piga:Acceleron Pharma: Research Funding; Novartis: Consultancy, Research Funding; Celgene Corporation: Consultancy, Research Funding. Forni:Roche, Erithropoiesis Stimulation: Research Funding; Celgene, Erithropoiesis Stimulation: Research Funding; Novartis, Iron chelation: Research Funding; BlueBirdBio: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal