Background: Anti-B cell maturation antigen (BCMA) chimeric antigen receptor(CAR) T cell therapy has shown promising results from a series of clinical trials. But short progression-free survival (PFS) due to BCMA-negative or positive relapse is pretty much the agenda.Here we constructed a dual-target BM38 CAR incorporating the anti-CD38 and anti-BCMA single-chain variable fragment in tandem plus 4-1BB signaling and CD3 zeta domains and conducted the first-in-human clinical trial(ChiCTR1800018143) in patients with RRMM to evaluate the safety, efficacy and duration of BM38 T cells.
Methods:Patients with relapsed or refractory multiple myeloma(RRMM), who had received at least 2 prior treatment regimens, including a proteasome inhibitor and an immunomodulatory agent, were enrolled in the phase 1 dose-climbing trial of the bispecific CAR-T cell therapy. Patients were subjected to a lymphodepleting regimen with Cy(250 mg/m2, d-5 to d-3) and Flu(25 mg/m2, d-5 to d-3) daily prior to the CAR-T infusion (d0). The dose gradients of infused CAR-T cells were 0.5, 1.0, 2.0, 3.0 and 4.0×106 cells/kg and at least 2 patients were involved at every dose level. The efficacy was assessed by the International Uniform Response Criteria for Multiple Myeloma (2016), and the toxicity was graded by CTCAE 5.0.
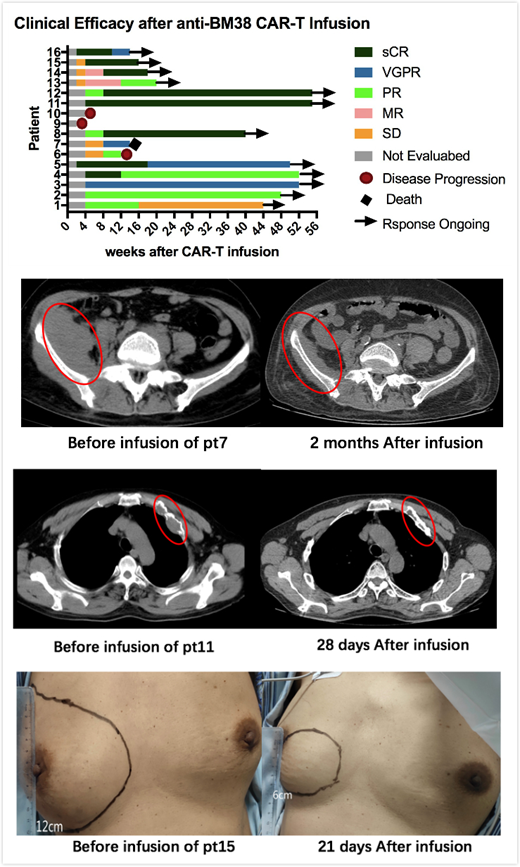
Results: As of 31 July 2019, 16 pts consisting of 10(62.5%) with genetic abnormalities and 5(31.25%) with extramedullary lesions,had received BM38 CAR-T cells in the 5 dose-climbing cohorts. At a median follow-up of 36 weeks, no DLTs and no grade ≥ 3 neurotoxicities were observed. Cytokine release syndrome (CRS), mainly grade 1-2, was reported in 10 of 16 (62.5%) pts; 4 pts had grade ≥ 3 CRS that resolved by tocilizumab and supportive treatment. Almost all the pts were observed with hematological toxicities relieved in the first month after infusion.14(87.5%) pts achieved an overall response with 8(50%) sCR, 2(12.5%) VGPR and 4(25.00%) PR and 14(87.5%) reached bone marrow minimal residual disease(MRD)-negative status. The longest duration of sCR was over 51 weeks and 5(62.5%) of 8 patients had still maintained sCR and 2 transformed to VGPR and 1 to PR. The median duration of progression-free survival(PFS) had not been reached; PFS rates at 9 months was 75%. More encouragingly, 5(100%)extramedullary lesions were eliminated.Up to the observed day, the BM38 CAR-T cells still exist in the patients' peripheral blood by flow cytometry(FCM) and quantitative polymerase chain reaction(q-PCR). The peak time of CAR-T cells proliferation of sCR patients was about the 2nd week after infusion, which was earlier than other patients. 4.0 × 106 CAR T cells (pt11, 12 and 15) were selected for the optimal dose with superior response and acceptable toxicities and expansion cohort would be conducted.
Conclusions:Our study demonstrates an improved efficacy with the bivalent BM38 CAR-T therapy for RRMM with a high ORR, especially a higher rate and a longer duration of sCR and effective elimination for extramedullary lesions. No neurotoxicity was observed. CRS and other toxicities were manageable. These initial data provide strong evidence to support the further development of the dual-target CAR-T therapy for RRMM.
Clinical trial information: ChiCTR1800018143
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal