
Introduction: Primary immune thrombocytopenia (ITP) is an acquired hemorrhagic disorder caused by immune-mediated platelet destruction and insufficient platelet production. As the first-line treatment of ITP, corticosteroids enable the recovery of platelet count in 60% to 80% of cases. However, a large proportion of patients would relapse during steroid tapering or withdrawal. Recently, sporadic studies reported successful treatment of ITP with oseltamivir which decreased platelet destruction through inhibition of platelet desialylation, a possible mechanism leading to Fc-independent platelet phagocytosis by hepatocytes. We hypothesize that corticosteroids and oseltamivir might complement each other in terms of mechanisms and induce a better response in ITP. Therefore, a prospective randomized controlled trial was conducted to compare the efficacy and safety of high-dose dexamethasone (HD-DXM) plus oseltamivir with HD-DXM monotherapy in adult ITP. Here we report the interim analysis of this study.
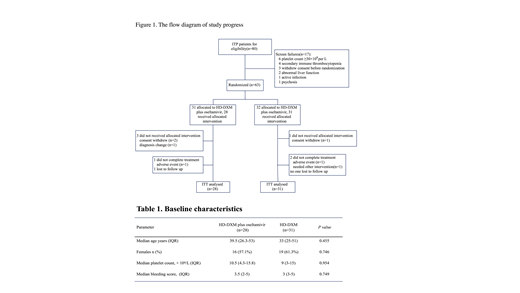
Methods: From February 2016 to May 2018, 80 newly diagnosed treatment-naïve ITP patients from 5 tertiary medical centers in China were screened in the trial (NCT01965626). Dexamethasone was administered orally at 40 mg daily to both arms for 4 days (days 1 - 4). If platelet counts remained < 30 × 109/L or there were bleeding symptoms by day 10, another course of HD-DXM was given (days 11 -14). For the combination arm, oral oseltamivir was given concomitantly at a dosage of 75 mg twice daily for the first 10 days (days 1 - 10). The primary endpoints were initial response and sustained response (SR). Platelet counts should be confirmed on two separate occasions at least 7 days apart when defining responses (CR: platelet count ≥ 100×109/L and absence of bleeding; R: platelet count ≥ 30 × 109/L but < 100 × 109/L and at least a doubling of baseline and absence of bleeding). Initial responses were assessed by day 14. Responders underwent follow-up for at least 12 months or until relapse. Response lasting for at least 6 consecutive months without additional ITP-specific intervention was regarded as SR. Secondary endpoints included time to response (TTR), bleeding scores, duration of response and adverse events (AEs). Platelet transfusion was permitted in patients with severe bleeding symptoms and recorded. Requirements for any additional ITP-specific intervention were considered as treatment failure.
Results: Fifty-nine patients were enrolled, of whom 28 were randomly to receive HD-DXM plus oseltamivir and 31 to HD-DXM monotherapy. Demographics and baseline characteristics were balanced between the 2 arms (Table 1). Results showed that HD-DXM plus oseltamivir achieved a higher incidence of initial response than HD-DXM alone at day 14 (82.1% vs. 58.1%, P = 0.045). At the end of 6 months, SR rate in the HD-DXM plus oseltamivir arm was higher than that in the HD-DXM monotherapy arm even though it did not reach statistical significance (42.8% vs. 25.8%, P = 0.167). Relapse-free survival in the HD-DXM plus oseltamivir arm was significantly improved compared with the HD-DXM monotherapy arm during follow-up period (HR 1.96, Log Rank P =0.043), as estimated by the Kaplan-Meier analysis. There was no statistical difference in median TTR between the two arms (P > 0.05). Bleeding was well controlled in both arms, with comparable post-treatment bleeding events (7.1% vs.19.3%, P> 0.05) and lower bleeding scores in the HD-DXM plus oseltamivir arm (0 vs. 0, P = 0.036). Incidence of AEs was similar between the 2 arms (P > 0.05). A slight increase in gastrointestinal reactions was observed in the HD-DXM plus oseltamivir arm but it did not reach statistical significance. Most of the AEs were mild to moderate (grade 1 to 2) and usually resolved spontaneously after medication was completed. No previously undescribed AEs was observed.
Conclusions: The combination of HD-DXM with oseltamivir significantly improved the initial response in newly diagnosed ITP patients. However, due to the limitation of sample size, the conclusion of SR superiority can hardly be drawn at the moment.
No relevant conflicts of interest to declare.
Oseltamivir, a sialidase inhibitor which is extensively used in the treatment of or prophylaxis against influenza virus A and B. It has been reported that the treatment of oseltamivir could concomitantly increase platelet counts, which was independent whether influenza was diagnosed. Along those lines, several studies showed successful treatment of ITP patients with oseltamivir. We hypothesize that HD-DXM and oseltamivir might complement each other in terms of the mechanisms and achieve better response rates in ITP patients.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal