
Background: The efficacy of the BCL2 inhibitor venetoclax combined with azacytidine (Ven+Aza), recently approved for elderly patients unfit for standard chemotherapy, highlights the promise of combination therapies for acute myeloid leukemia (AML). However, overall and progression-free survival remain at ~30-40% after 1 year of therapy, warranting exploration of new venetoclax combinations and elucidation of mechanisms and biomarkers of response and resistance.
Methods: We examined a series of venetoclax combinations using a functional genomics approach applied directly to >350 primary AML patient specimens. Primary patient mononuclear cells were plated ex vivo with a panel of 10 venetoclax combinations and the represented single agents. Whole exome and RNA-sequencing were obtained from the same patient samples. Levels of plasma cytokine levels were obtained from the patient samples where possible. Clinical, genetic, and disease status information were collated, and ex vivo combination sensitivities were compared to matched single agent data by Friedman test, across groups by Kruskal-Wallis test, and with continuous variables by Spearman rank correlation.
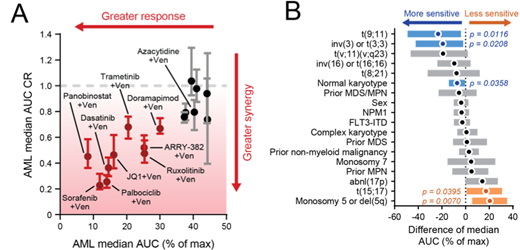
Results: Consistent with clinical findings, Ven+Aza showed significantly enhanced efficacy in AML samples ex vivo (median IC50 = 0.100 uM), with evidence of enhanced efficacy compared to each single agent (AUC CR = 0.795). Using this as a benchmark, we found 9 venetoclax combinations (venetoclax paired with ruxolitinib, palbociclib, ARRY-382, doramapimod, trametinib, sorafenib, dasatinib, JQ1, or panobinostat) which exceeded both the ex vivo activity and enhanced efficacy observed with Ven+Aza (Fig. 1A). We evaluated associations of clinical and genetic data with combination sensitivity, using the Rux+Ven combination as a lead example. Importantly, Rux+Ven demonstrated similarly enhanced ex vivo efficacy in newly diagnosed and relapsed/refractory AML patient specimens. At the genetic level, Rux+Ven sensitivity included not only specimens harboring the highly frequent FLT3-ITD and NPM1 mutations, but also those with TP53 mutations, which carries a very poor disease prognosis clinically and associated with venetoclax resistance. Certain cytogenetic features correlated with greater sensitivity to Rux+Ven (e.g. t(9;11) - MLL-rearrangement) or decreased sensitivity (e.g. t(15;17) - PML-RARA or monosomy 5/del 5q) (Fig. 1B). To define gene expression-based differences associated with ex vivo sensitivity to venetoclax combinations, we assessed expression level differences between the 25% most sensitive and 25% least sensitive patient samples. For the Rux+Ven combination, this yielded ~1500 differentially expressed genes (FDR-adjusted p-value < 0.05) which showed enrichment of several Reactome pathways including PD-1 and IL-27 signaling, suggestive of a role for cytokine and immune signals. Concordantly, we found levels of certain cytokines correlated with Rux+Ven sensitivity or resistance. Similarly analyses of clinical and genetic biomarkers of sensitivity and resistance for the other venetoclax combinations tested is in progress and will be presented.
Conclusion: We have identified drug combinations with ex vivo activity that exceeds that of Ven+Aza and have identified clinical, mutational, and expression-based biomarker patterns associated with sensitivity and resistance to these combinations. Importantly, an understanding of the biology underlying the efficacy of each specific combination will offer an opportunity to fine-tune their clinical application towards the AML subsets most likely to benefit from each combination strategy and may also propel testing of these combinations in other malignancies.
Figure 1. Sensitivity of Venetoclax-containing combinations on AML patient samples.A. Summary of all venetoclax combinations tested for efficacy (AUC, horizontal axis) and synergy (CR vertical axis). CR denotes Combination Ratio and is defined as the AUC of the combination/AUC of the most effective single agent in the combination. Median AUC CR and 95% CI are shown. Red indicates combinations with better efficacy and synergy than venetoclax + azacytidine B. Summary of clinical and genetic features of AML that correlate with Rux+Ven sensitivity (blue) or resistance (orange). **** denotes p<0.0001; *** p<0.001; * p<0.05; n.s., not significant.
Druker:Pfizer: Other: PI or co-investigator on clinical trial(s) funded via contract with OHSU., Research Funding; Bristol-Myers Squibb: Patents & Royalties, Research Funding; OHSU (licensing fees): Patents & Royalties: #2573, Constructs and cell lines harboring various mutations in TNK2 and PTPN11, licensing fees ; Dana-Farber Cancer Institute (antibody royalty): Patents & Royalties: #2524, antibody royalty; Merck & Co: Patents & Royalties: Dana-Farber Cancer Institute license #2063, Monoclonal antiphosphotyrosine antibody 4G10, exclusive commercial license to Merck & Co; The RUNX1 Research Program: Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; Aileron Therapeutics: #2573, Constructs and cell lines harboring various mutations in TNK2 and PTPN11, licensing fees , Membership on an entity's Board of Directors or advisory committees; Vivid Biosciences: Membership on an entity's Board of Directors or advisory committees, Other: Stock options; ALLCRON: Membership on an entity's Board of Directors or advisory committees; Amgen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; GRAIL: Equity Ownership, Other: former member of Scientific Advisory Board; Patient True Talk: Consultancy; Blueprint Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Burroughs Wellcome Fund: Membership on an entity's Board of Directors or advisory committees; Cepheid: Consultancy, Honoraria; Aptose Biosciences: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Beta Cat: Membership on an entity's Board of Directors or advisory committees, Other: Stock options; Monojul: Other: former consultant; CureOne: Membership on an entity's Board of Directors or advisory committees; Beat AML LLC: Other: Service on joint steering committee; Bristol-Myers Squibb: Other: PI or co-investigator on clinical trial(s) funded via contract with OHSU., Research Funding; Novartis: Other: PI or co-investigator on clinical trial(s) funded via contract with OHSU., Patents & Royalties: Patent 6958335, Treatment of Gastrointestinal Stromal Tumors, exclusively licensed to Novartis, Research Funding; ICON: Other: Scientific Founder of Molecular MD, which was acquired by ICON in Feb. 2019; Gilead Sciences: Other: former member of Scientific Advisory Board; Celgene: Consultancy. Tyner:Syros: Research Funding; Petra: Research Funding; Takeda: Research Funding; Aptose: Research Funding; Gilead: Research Funding; Incyte: Research Funding; AstraZeneca: Research Funding; Incyte: Research Funding; Constellation: Research Funding; Genentech: Research Funding; AstraZeneca: Research Funding; Aptose: Research Funding; Seattle Genetics: Research Funding; Petra: Research Funding; Janssen: Research Funding; Constellation: Research Funding; Syros: Research Funding; Seattle Genetics: Research Funding; Takeda: Research Funding; Array: Research Funding; Janssen: Research Funding; Agios: Research Funding; Agios: Research Funding; Array: Research Funding; Gilead: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal