Introduction: Deferasirox (DFX) is the currently available iron-chelation therapy (ICT) for the management of iron overload (IOL), mainly in myelodysplastic syndromes; recently, two retrospective independent studies (Di Veroli, 2018; Elli, 2019) demonstrated that a treatment with DFX is feasible and effective also in the setting of myelofibrosis (MF). However, no data are still available regarding the concomitant use of DFX in patients (pts) treated with Ruxolitinib (Rux).
Aims and Methods: We retrospectively collected in 16 Italian Centers 59 pts (M: 37; F: 22) with primary MF (n=41) or post-Polycythemia Vera (n=9) and post-Essential Thrombocythemia (n=9) MF, treated with Rux and DFX for the management of IOL secondary to transfusion-dependent anemia. Primary endpoint of the study was to evaluate the efficacy of DFX in terms of reduction of ferritin levels and hematological improvement (HI). Additional endpoints were the safety of DFX associated to Rux treatment and the impact on survival and leukemic evolution.
Results: The main features of pts at diagnosis and at baseline of DFX treatment are reported in Table 1 (column A). Pts started DFX after a median time from MF diagnosis of 26.7 months [Interquartile range (IR) 2.6-240.9] and from transfusion dependency of 13.5 months (IR 1.5 - 145.3). Forty-eight pts started DFX when already under Rux treatment, while 11 pts before Rux treatment. The median ferritin level (FL) at baseline was 1675 ng/mL (IR 646-6447). The median starting dose of DFX was 1000 mg/day (12.5 mg/kg/day).
All pts were evaluable for DFX response (> 3 months of DFX), with a median time of DFX and Rux exposure of 14.5 months (IR 3.2-73.3) and 40 months (2.1-88.6), respectively; the median period of concomitant DFX-Rux treatment was 11.1 months (3.7-58.4). As to ICT efficacy, 24 pts (40.7%) obtained an iron chelation response (ICR), defined as a stable reduction of FL < 1.000 ng/mL or a reduction ≥ 50% of FL respect to baseline. The main variables analyzed in pts with ICR or no ICR were reported in Table 1 (Column B and C, respectively). At univariate analysis, pts who obtained ICR did not presented significant differences compared to pts without ICR, except for a significantly lower median FL at diagnosis (251 vs 496 mcg/l, p=0.008). As expected, ICR pts showed a progressive significant reduction of FL at 3, 6, 12 and 18 months, respect to baseline, in contrast to pts without ICR (p < 0.0001). The median time of exposure to DFX was higher in ICR vs no ICR group (22.0 vs 12.9 months, p=0.03), as well as the median time of concomitant DFX-Rux treatment (14.2 vs 9.7 months, p=0.019).
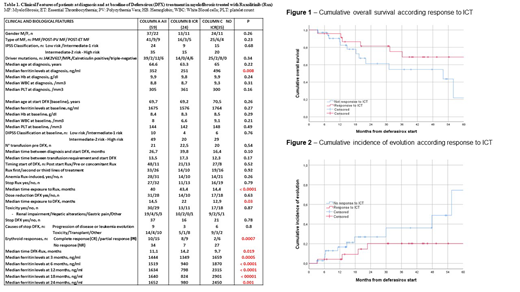
The International Working Group criteria (Cheson, 2006) were applied to assess HI during ICT. Erythroid response (ER) was defined as complete (CR: achievement of transfusion independency), partial (PR: reduction in the transfusion requirement ≥ 50% and/or increase of haemoglobin levels) or no response (NR). ER was observed in 25 pts (42.4%) with ten (17%) obtaining CR, 15 (25.4%) PR and 34 (57.6%) NR. Obtainment of ICR predicted for the achievement of ER: 17 (70.8%) pts with ICR achieved CR or PR compared to 8 (22.8%) without ICR (p =0.0007). DFX-related toxicities occurred in 30 pts (50.8%) and consisted mainly in renal impairment (32.2%), liver enzymes alterations (6.7%) or epigastric pain (8.4%): however, only in one case was observed a grade 3 toxicity. Overall, a dose reduction/temporary discontinuation related to DFX-toxicity was reported in 14 (23.7%) pts; however only 5 (8.4%) pts completely discontinued ICT because of grade ≥ 2 toxicity. After a median follow-up from diagnosis of 58.6 months (IR 7.1-282.9), 19 pts (32.2%) died [13 of them (22%) for leukemic evolution or disease progression]. The 3-year cumulative overall survival (Figure 1) and the 3-year cumulative incidence of leukemic evolution (Figure 2) from DFX initiation were 75.1% (95%CI 55.6 - 94.6) and 20.2% (95%CI 2.1 - 38.3) in pts who obtained ICR compared to 54.5% (95%CI 31.9 - 77.1) and 36.1% (95%CI 13.1 - 59.1) in pts without ICR, respectively (p=0.13 and p=0.153).
Conclusions: The present multicenter study showed that ICT with DFX is effective and safe also in this setting of pts receiving concomitant Rux treatment. HI with ER seems occur in a significant proportion of pts and correlates with the achievement of ICR. Further larger and prospective investigations are required in order to evaluate the impact on survival and leukemic evolution of this combination.
Elli:Novartis: Membership on an entity's Board of Directors or advisory committees. Iurlo:Novartis: Other: Speaker Honoraria; Incyte: Other: Speaker Honoraria; Pfizer: Other: Speaker Honoraria. Benevolo:Novartis Pharmaceuticals: Consultancy. Abruzzese:BMS: Consultancy; Incyte: Consultancy; Novartis: Consultancy; Pfizer: Consultancy. Bonifacio:Novartis: Honoraria; Amgen: Honoraria; BMS: Honoraria; Pfizer: Honoraria; Incyte: Honoraria. Cilloni:Janssen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Tiribelli:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Trawinska:Novartis: Consultancy, Honoraria. Breccia:BMS: Honoraria; Pfizer: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Celgene: Honoraria. Gambacorti-Passerini:Bristol-Meyers Squibb: Consultancy; Pfizer: Honoraria, Research Funding. Palumbo:Janssen: Honoraria; Celgene: Honoraria; Teva: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Hospira: Honoraria. Latagliata:Celgene: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal