
Background: Loss of immune surveillance, mediated through immune checkpoint (ICP) interactions, is thought to be a key step in the development of cancers including AML and HR-MDS. AZA is a standard therapy for pts with AML who are unfit for IC and for pts with HR-MDS. AZA can promote immune recognition of tumor cells and potentially increase expression of ICP molecules, which can mediate resistance to AZA. As myeloid cell lines and samples from pts treated with hypomethylating agents demonstrated up-regulation of PD-L1 expression, blockade of the PD-L1 ICP with durva in combination with AZA may enhance antitumor activity and improve clinical outcomes. Here, we report the final results from a large phase 2 study evaluating the efficacy and safety of AZA+durva vs. AZA alone in pts with HR-MDS or AML (NCT02775903).
Methods: This randomized, open-label, international, multicenter study enrolled untreated pts in 2 cohorts: 1) MDS (aged ≥18 years; IPSS-R intermediate, high, and very high) and 2) older AML pts (aged ≥65 years) who were ineligible for IC. All pts had ECOG performance status 0-2 and were separately randomized (1:1) to receive SC AZA 75 mg/m2 Days 1-7 and durva 1500 mg IV on Day 1 Q4W (Arm A) or AZA alone (Arm B) and stratified according to cytogenetic risk (MDS, very good/good/intermediate vs. poor/very poor; AML, intermediate vs. poor). Treatment was planned to continue until progression or unacceptable toxicity. Disease status was evaluated every third treatment cycle. Primary MDS endpoints included overall response rate (ORR, defined as complete remission [CR], marrow [m]CR, partial response [PR], or hematologic improvement [HI]) based on IWG 2006 response criteria, while for AML ORR was defined as CR or CR with incomplete blood recovery (CRi) based on modified IWG 2003 response criteria. Secondary endpoints included PFS, OS, and safety. Peripheral blood samples were collected to assess changes in DNA methylation using the EPIC methylation array (Illumina). Bone marrow (BM) aspirates were obtained for quantitation of PD-L1 surface expression by flow cytometry and values are reported as molecules of equivalent soluble fluorochrome.
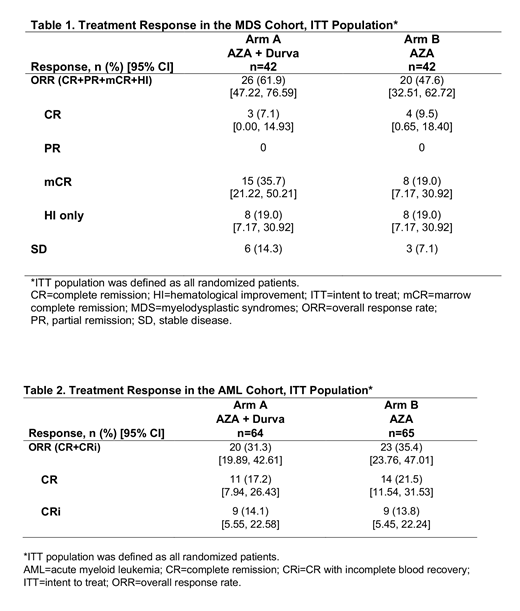
Results: A total of 213 pts, 84 with MDS (each arm, n=42) and 129 with AML (Arm A, n=64; Arm B, n=65) were randomized. As of October 31, 2018, 32 pts (MDS, n=14; AML, n=18) continued to receive trial treatment while 181 (MDS, n=70; AML, n=111) had discontinued. Baseline demographics and disease characteristics were generally balanced across treatment groups in both cohorts. Median number of treatment cycles for AML Arm A vs. B, 6.5 vs. 6.7; for MDS Arm A vs. B, 7.9 vs. 7.0. No statistically significant differences in ORR between treatment arms were observed in either cohort (Tables 1 and 2). In MDS Arm A vs. B, median OS was 11.6 vs. 16.7 months (mo) and PFS was 8.7 vs. 8.6 mo. In the AML cohort, median OS was 13.0 vs. 14.4 mo and PFS was 8.1 vs. 7.2 mo. Caution should be used when interpreting results because >50% of patients were censored. The most frequent TEAEs (≥15%) were hematologic and GI toxicity. In the MDS and AML cohorts, 7 and 17, respectively, immune-mediated AEs were observed; all were treated and resolved. AZA induced similar trends in global hypomethylation, along with focal hypomethylation of PD-L1 and PD-L2 gene loci, at the end of treatment cycle 1 in all treatment groups and cohorts. Mean PD-L1 surface expression in BM immune cells at baseline was highest in monocytes (MDS=1,425; AML=1,536), followed by granulocytes (MDS=550; AML=758) and myeloid blasts (MDS=532; AML=735). Increased surface expression of PD-L1, but not PD-L2, was observed at the end of treatment cycle 3 on BM granulocytes and monocytes from MDS pts and on BM monocytes from AML pts, but no increase was detected on myeloid blasts.
Conclusions: To our knowledge, this is the first large randomized trial of AZA with or without ICP blockade in older unfit AML and HR-MDS pts reported to date. No clinically meaningful difference in efficacy was observed between treatments for either cohort. No new safety signals or potential overlapping risks were identified with the combination. While the hypomethylating activity of AZA on PD-L1 gene was confirmed, no treatment-mediated induction of PD-L1 surface expression was observed on myeloid blasts.
Zeidan:Acceleron Pharma: Consultancy, Honoraria, Research Funding; Celgene Corporation: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Otsuka: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Medimmune/AstraZeneca: Research Funding; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Trovagene: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Research Funding; Jazz: Honoraria; Ariad: Honoraria; Agios: Honoraria; Novartis: Honoraria; Astellas: Honoraria; Daiichi Sankyo: Honoraria; Cardinal Health: Honoraria; Seattle Genetics: Honoraria; BeyondSpring: Honoraria. Voso:Novartis: Speakers Bureau; Celgene: Research Funding, Speakers Bureau. Taussig:Celgene: Research Funding. Boss:Celgene Corporation: Employment, Equity Ownership. Copeland:Celgene Corporation: Employment, Equity Ownership. Gray:Celgene Corporation: Employment, Equity Ownership. Previtali:Celgene Corporation: Employment, Equity Ownership. O'Connor:Celgene Corporation: Employment, Equity Ownership. Rose:Celgene Corporation: Employment, Equity Ownership. Beach:Celgene Corporation: Employment, Equity Ownership.
Durvalumab is a PD-L1 blocking antibody indicated for the treatment of patients with 1) locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy, or who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy, or 2) unresectable, stage 3 NSCLC whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal