
Introduction: Anti-PD-1 monoclonal antibodies (mAbs) are highly active in relapsed/refractory classical Hodgkin lymphoma (cHL), but most patients (pts) will still relapse. Given this, allogeneic stem cell transplantation (alloHSCT) remains an important option for pts after PD-1 blockade, as it offers the possibility of cure. Prior reports have suggested that alloHSCT after PD-1 mAbs may be associated with severe immune-related complications including acute graft-versus-host disease (GVHD), veno-occlusive disease (VOD) and cytokine release/febrile non-infectious syndrome (CRS). Prior studies of alloHSCT after PD-1 blockade in cHL have been limited by the small number of pts and short follow-up, preventing an accurate assessment of long-term outcomes and complications, risk factors for early toxicity, and the impact of transplant strategies such as choice of GVHD prophylaxis. We therefore assembled a large retrospective international cohort of cHL pts who underwent alloHSCT after PD-(L)1 blockade to better answer these questions, including an assessment of the impact of post-transplant cyclophosphamide (PTCy) on efficacy and toxicity.
Methods: Medical records and databases were reviewed at 26 European and United States transplant centers to identify pts with cHL who underwent an alloHSCT any time after receiving a PD-1 or PD-L1 mAb. Response assessment was performed by local investigators according to Lugano 2014 criteria. Overall survival (OS), progression-free survival (PFS), cumulative incidence (CumInc) of relapse (CIR), non-relapse mortality (NRM), acute (a) and chronic (c) GVHD were estimated, as was the association between baseline variables and these outcomes.
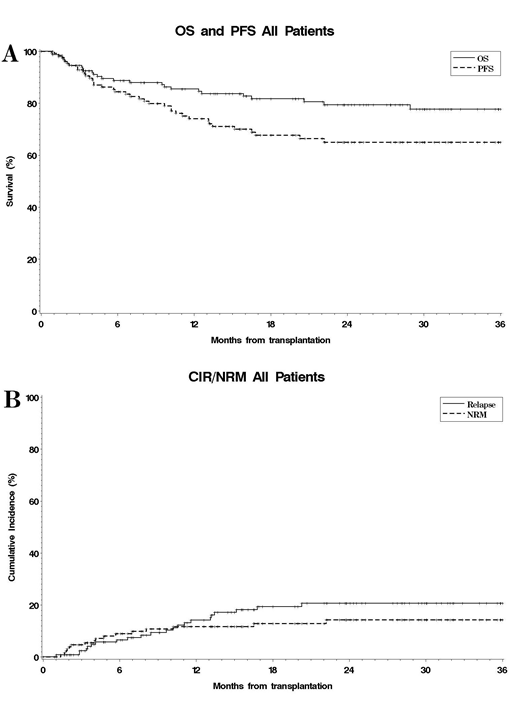
Results: Between 2014 and 2019, 150 pts were identified who underwent alloHSCT after a median of 10 (range, 1-74) doses of nivolumab (n=118), pembrolizumab (n=31), or avelumab (n=1). The median age was 31 (range 17-68) and pts had received a median of 4 (range, 2-11) lines of therapy prior to PD-(L)1 blockade. 138 pts (92%) had failed BV and 111 (74%) autologous HSCT. The best overall response to PD-(L)1 mAbs was CR for 62 pts (41%), PR for 55 (37%), SD for 17 (11%), PD for 15 (10%) and unknown for 1 (1%). Median time from last dose of PD-(L)1 mAb to alloHSCT was 80 days (range, 17-756) with 70 pts (47%) receiving intervening systemic therapy. At alloHSCT, 90 pts were in CR (60%), 45 in PR (30%), 5 in SD (3%), and 10 in PD (7%). Donors were haploidentical (n=71, 47%), matched sibling (n=29, 19%), matched unrelated (n=39, 26%), mismatched unrelated (n=7, 5%), cord blood (n=2, 1%), or unknown (n=2, 1%). Stem cell source was bone marrow (n=38, 25%), peripheral blood (n=110, 73%), or cord blood (n=2, 1%). GVHD prophylaxis included PTCy in 88 pts (59%) (69/71 (97%) with haploidentical donors; 19/79 (24%) with other donors). With a median post-alloHSCT follow-up for survivors of 23.8 months (range, 1-67), the 2y OS and PFS were 79% (95CI 71-86%) and 65% (95CI 55-73%), respectively, while the 2y CumIncs of relapse and NRM were 21% (95CI 13-29%) and 14%, (95CI, 8-22%), respectively (Fig. 1A-B). 27 pts have died, 3 due to disease and 24 to NRM, including aGVHD (n=7) and VOD (n=2). Veno-occlusive disease (VOD) occurred in 5 pts (day 100 CumInc 4%) and 29 pts (19%) developed CRS (grade 1 n=16; grade 2 n=7; grade 3 n=4; grade 4 n=2). The 6-month CumIncs of grade 2-4, grade 3-4 and grade 4 aGVHD were 39%, 16% and 8%, respectively. Hyperacute GVHD (onset ≤ 14 days after alloHSCT) occurred in 4% of pts and was fatal in 2 pts. The 2y CumInc of cGVHD was 45%. Neither receipt of > 10 doses (median) of anti-PD-(L)1 mAb nor undergoing alloHSCT ≤80 days (median) after last dose of PD-(L)1 mAb were associated with PFS or OS. However, pts with a shorter time to transplant (≤80 days) appeared to have a higher risk of severe (grade 3-4) aGVHD (6m CumInc 24% vs 9%, p=0.006). Recipients of PTCy in this cohort had lower 2y CumIncs of cGVHD (34% vs 58%, p=0.01) and relapse (12% vs 31%, p=0.02), superior 2y PFS (76% vs 54%, p=0.015), and similar rates of severe aGVHD (15% vs 18%, p=0.5), 2y NRM (12% vs 16%, p=0.5), and 2y OS (82% vs 78%, p=0.6).
Conclusions: With extended follow-up of a large international cohort, our results argue that alloSCT performed after PD-(L)1 mAbs is a feasible strategy associated with an excellent PFS and a very low CIR for this disease. The use of PT-Cy appears to be associated with improved outcomes and may at present represent the optimal transplant strategy in this pt population.
Corradini:kite: Honoraria; Abbvie: Honoraria; Servier: Honoraria; Sanofi: Honoraria; Takeda: Honoraria; Roche: Honoraria; Novartis: Honoraria; KiowaKirin: Honoraria; Janssen: Honoraria; Gilead: Honoraria; Daiichi Sankyo: Honoraria; Celgene: Honoraria; Amgen: Honoraria. Ho:Jazz Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Consultancy; Omeros Corporation: Membership on an entity's Board of Directors or advisory committees. Jaglowski:Kite: Consultancy, Other: advisory board, Research Funding; Novartis: Consultancy, Other: advisory board, Research Funding; Juno: Consultancy, Other: advisory board; Unum Therapeutics Inc.: Research Funding. Herrera:Adaptive Biotechnologies: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Gilead Sciences: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; AstraZeneca: Research Funding; Merck: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding; Pharmacyclics: Research Funding; Immune Design: Research Funding; Kite Pharma: Consultancy, Research Funding. Blaise:Jazz Pharmaceuticals: Honoraria; Sanofi: Honoraria; Pierre Fabre medicaments: Honoraria; Molmed: Consultancy, Honoraria. Hamadani:Pharmacyclics: Consultancy; Sanofi Genzyme: Research Funding, Speakers Bureau; Merck: Research Funding; Otsuka: Research Funding; Janssen: Consultancy; Medimmune: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Takeda: Research Funding; Celgene: Consultancy. Ansell:LAM Therapeutics: Research Funding; Affimed: Research Funding; Affimed: Research Funding; Trillium: Research Funding; Trillium: Research Funding; Seattle Genetics: Research Funding; Mayo Clinic Rochester: Employment; Mayo Clinic Rochester: Employment; Bristol-Myers Squibb: Research Funding; Affimed: Research Funding; Mayo Clinic Rochester: Employment; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding; LAM Therapeutics: Research Funding; Mayo Clinic Rochester: Employment; Seattle Genetics: Research Funding; LAM Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; Trillium: Research Funding; Trillium: Research Funding; Bristol-Myers Squibb: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Mayo Clinic Rochester: Employment; Bristol-Myers Squibb: Research Funding; Regeneron: Research Funding; Regeneron: Research Funding; Regeneron: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; Trillium: Research Funding; LAM Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; Mayo Clinic Rochester: Employment; Regeneron: Research Funding; Trillium: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; LAM Therapeutics: Research Funding; LAM Therapeutics: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; LAM Therapeutics: Research Funding; Trillium: Research Funding; Trillium: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Trillium: Research Funding; Affimed: Research Funding. Nieto:Astra-Zeneca: Research Funding; Affimed: Consultancy; Affimed: Research Funding; Novartis: Research Funding. Feldman:Celgene: Honoraria, Research Funding, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Other: Travel expenses, Speakers Bureau; Pfizer: Research Funding; AbbVie: Honoraria, Other: Travel expenses, Speakers Bureau; Portola Pharma: Research Funding; Kite Pharma: Honoraria, Other: Travel expenses, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Pharmacyclics: Honoraria, Other: Travel expenses, Speakers Bureau; Kyowa Hakko Kirin: Research Funding; Eisai: Research Funding; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Cell Medica: Research Funding; Roche: Research Funding; Corvus: Research Funding; Viracta: Research Funding; Trillium: Research Funding; Roche: Research Funding; Takeda: Honoraria, Speakers Bureau. McGuirk:ArticulateScience LLC: Other: Assistance with manuscript preparation; Bellicum Pharmaceuticals: Research Funding; Kite Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gamida Cell: Research Funding; Pluristem Ltd: Research Funding; Juno Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Research Funding; Novartis: Research Funding; Fresenius Biotech: Research Funding. Mohty:Jazz Pharmaceuticals: Honoraria, Research Funding. Stamatoulas Bastard:Celgene: Honoraria; Takeda: Consultancy. Houot:Bristol Myers Squibb: Honoraria; Merck Sharp Dohme: Honoraria. Manson:Bristol Myers Squibb: Honoraria. Orvain:Incyte: Honoraria; Novartis: Honoraria; Jazz Pharmaceuticals: Other: Travel & accommodations; Pfizer: Other: Travel & accommodations. Bouabdallah:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Frigault:Novartis: Consultancy; Kite/Gilead: Honoraria; Nkarta: Consultancy; Incyte: Consultancy; Juno/Celgene: Consultancy; Foundation Medicine: Consultancy; Xenetic: Consultancy. Chen:Takeda: Consultancy; Kiadis: Consultancy; Magenta: Consultancy; Abbvie: Consultancy; Incyte: Consultancy. Lynch:T.G. Therapeutics: Research Funding; Rhizen Pharmaceuticals S.A: Research Funding; Takeda Pharmaceuticals: Research Funding; Juno Therapeutics: Research Funding; Incyte Corporation: Research Funding; Johnson Graffe Keay Moniz & Wick LLP: Consultancy. Smith:AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck Sharp & Dohme Corp: Consultancy, Research Funding; Acerta Pharma BV: Research Funding; Portola Pharmaceuticals: Research Funding; Pharmacyclics: Research Funding; Bristol-Myers Squibb (spouse): Research Funding; Denovo Biopharma: Research Funding; Genentech: Research Funding; Ignyta (spouse): Research Funding; Incyte Corporation: Research Funding; Ayala (spouse): Research Funding; Seattle Genetics: Research Funding. Byrne:Karyopharm: Research Funding. Cohen:Hutchison: Research Funding; Astra Zeneca: Research Funding; Janssen Pharmaceuticals: Consultancy; Seattle Genetics, Inc.: Consultancy, Research Funding; Bristol-Meyers Squibb Company: Research Funding; Takeda Pharmaceuticals North America, Inc.: Research Funding; Gilead/Kite: Consultancy; Genentech, Inc.: Consultancy, Research Funding; UNUM: Research Funding; ASH: Research Funding; LAM Therapeutics: Research Funding; Lymphoma Research Foundation: Research Funding. Svoboda:AstraZeneca: Consultancy; Celgene: Research Funding; Incyte: Research Funding; Pharmacyclics: Consultancy, Research Funding; Kyowa: Consultancy; Merck: Research Funding; BMS: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding. Santoro:Bayer: Consultancy, Speakers Bureau; MSD: Speakers Bureau; Arqule: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; AstraZeneca: Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Servier: Consultancy, Speakers Bureau; Takeda: Speakers Bureau; BMS: Speakers Bureau; Roche: Speakers Bureau; Abb-Vie: Speakers Bureau; Amgen: Speakers Bureau; Celgene: Speakers Bureau; Novartis: Speakers Bureau; BMS: Consultancy; Lilly: Speakers Bureau; Sandoz: Speakers Bureau; Eisai: Consultancy, Speakers Bureau. Armand:Sigma Tau: Research Funding; Otsuka: Research Funding; Pfizer: Consultancy; ADC Therapeutics: Consultancy; Tensha: Research Funding; Affimed: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; Adaptive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Infinity: Consultancy; Genentech: Research Funding. Zinzani:Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Portola: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Immune Design: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Honoraria, Speakers Bureau. Carlo-Stella:Servier: Consultancy, Honoraria, Other: Travel, accommodations; Genenta Science srl: Consultancy; Boehringer Ingelheim: Consultancy; F. Hoffmann-La Roche Ltd: Honoraria, Other: Travel, accommodations, Research Funding; Novartis: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Other: Travel, accommodations, Research Funding; Sanofi: Consultancy, Research Funding; Rhizen Pharmaceuticals: Research Funding; Celgene: Research Funding; Amgen: Honoraria; AstraZeneca: Honoraria; Janssen Oncology: Honoraria; MSD: Honoraria; BMS: Honoraria; Janssen: Other: Travel, accommodations; Takeda: Other: Travel, accommodations.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal