PURPOSE
In diffuse large B cell lymphoma (DLBCL), surviving disease free for 2 years after immunochemotherapy is associated with a normal life expectancy (Maurer, et al JCO 2014; Jakobsen et al, JCO 2017). Here we examine the conditional survival and standardized mortality ratio (SMR) among patients with relapsed de novo DLBCL successfully undergoing an autologous stem cell transplant (ASCT) for primary refractory or relapsed disease.
PATIENTS AND METHODS
A total of 478 patients with de novo DLBCL, progressing or relapsed after one treatment regimen from CORAL (Gisselbrecht et al, JCO 2010) or LY.12 (Crump et al, JCO 2013), were included in this analysis. These studies tested two salvage regimens (R-ICE or R-GDP) against R-DHAP pre-ASCT, and maintenance rituximab post-ASCT. Patients were followed prospectively after ASCT for a median of 5.3 and 8.2 years, respectively. Individual patient data were analysed for event free survival (EFS) and overall survival (OS). As well, standardized mortality ratios (SMR) were estimated using French and Canadian lifetables.
RESULTS
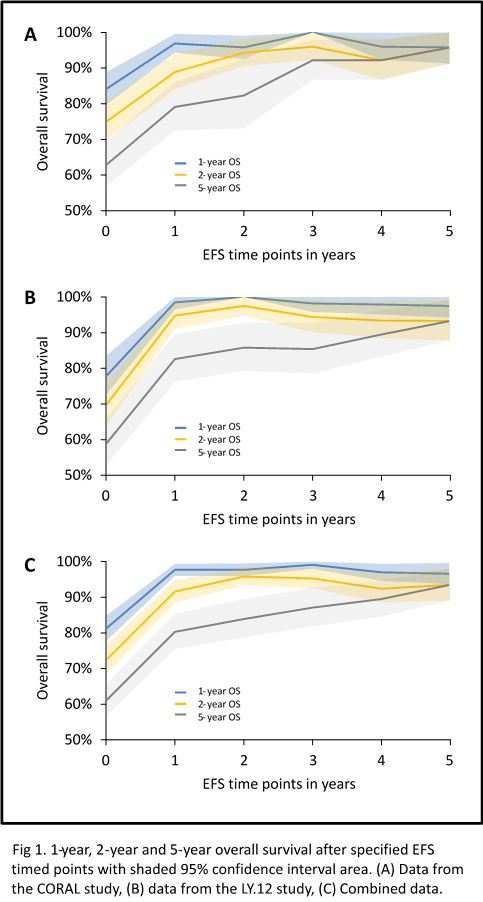
The number of patients alive and event free (EFS) 2 years after ASCT was 55.6% for patients treated in CORAL, 54.8% for LY.12, and 55.2% for the entire cohort (Figure). At 5 years, 33.5% and 39.4% are alive without event for CORAL and LY.12, respectively and 36.2% for the entire cohort. Patients who achieve EFS24 have an overall survival of 82.3% for CORAL and 85.8 % for LY.12 at 5 years. Compared with the age and sex matched population, the standardized mortality ratio (SMR) was significantly higher until 5 years after ASCT, when there is no longer a statistically significant difference, SMR is 4.5 (95% CI 0.9-13.3) for CORAL and 2.3 (95% CI 0.8-5.0) for LY.12. Causes of death are dominated by ongoing lymphoma relapse.
CONCLUSION
Patients undergoing ASCT for relapsed DLBCL who achieve EFS24 have a very good long-term survival rate but continue to have a higher rate of death than the general population at least until they have survived disease free for 5 years. These observations can help to determine endpoints for clinical trials of new agents and approaches in this population, and in discussing outcomes with patients referred for ASCT.
Assouline:Janssen: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Speakers Bureau. Hay:MorphoSys: Research Funding; Gilead: Research Funding; Kite: Research Funding; Takeda: Research Funding; AbbVie: Research Funding; Seattle Genetics: Research Funding; Celgene: Research Funding; Novartis: Research Funding; Janssen: Research Funding; Roche: Research Funding. Van Den Neste:Gilead: Other: travel support. Schmitz:Riemser: Consultancy, Honoraria; Celgene: Equity Ownership; Gilead: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal