Background: MCL is a heterogeneous disease and the existence of indolent clinical forms is increasingly recognized although their biological ground is not fully elucidated. The aim of this study was to propose a frontline tailored treatment for indolent clinical forms with a chemo-free regimen, ibrutinib in combination with rituximab. In addition, an extensive genomic study was associated to gain biological insight into these clinical forms.
Methods: This is a multicenter single-arm, open-label, phase II study with a two-stage design conducted in 14 Spanish GELTAMO sites (NCT02682641). Centralized histology, PET-CT review as well as minimal residual disease (MRD) studies (qPCR and NGS in peripheral blood [PB] and bone marrow [BM]) and biological studies are conducted. A total of 50 previously untreated MCL patients with indolent clinical forms are planned to be recruited, defined by the following criteria: no symptoms attributable to MCL, ECOG 0-1, stable disease without therapy need at least for 3 months, non-blastoid variants, Ki-67 <30% and largest tumor diameter ≤3 cm. Both leukemic non-nodal and nodal forms were acceptable. Patients received ibrutinib 560 mg daily and a total of 8 doses of Rituximab 375 mg/m2 (4 weekly doses during first 28-day cycle, followed by day 1 of cycles 3, 5, 7 and 9). Ibrutinib could be discontinued after 2 years of treatment in case of negative MRD for at least 6 months. The primary endpoint was the rate of complete remission (CR) achieved after 12 cycles according to the Lugano criteria (Cheson, 2014).
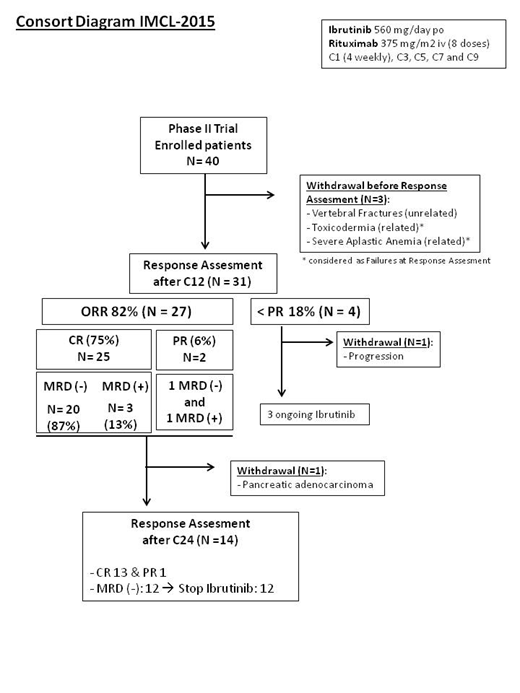
Results: Forty patients (Gender M 29/ F 11; median age 65.7 years; low-risk MIPI 22% and intermediate/high MIPI 78%) were enrolled in the study up to data cut-off on 15 MAY 2019 (Consort diagram). The median observation time for the patients before treatment was of 7.6 months (range:3-107) and median follow-up was of 19 months. Efficacy data of the first 33 patients evaluable after 12 cycles of treatment are reported here, including two patients who were discontinued before cycle 12 due to related toxicity. A total of 27 patients achieved a response with an 82% overall response rate (ORR) and 75% CR. Among CR patients with evaluable MRD (N=23), the rate of undetectable MRD achieved after 12 cycles was 87%. Only 1 patient eventually became MRD positive at cycle 24, whereas 12 remained MRD negative and accordingly, nine of them discontinued ibrutinib as per protocol whereas 3 had interrupted treatment earlier because of intolerance. At data cut-off, all responding patients maintained the response with a median follow-up of 25 months (12-35). Only one patient progressed at one year of therapy. This particular case had shown intolerance to full-dose ibrutinib, received different salvage therapies and died of progressive disease. The estimated 15 month progression-free survival was 96% (CI95%: 89-100). Four patients withdrew the study because of serious adverse events, including cutaneous rash, severe aplastic anemia, pancreatic adenocarcinoma and lumbar fractures. Twenty-one additional G3 and G4 AEs related to Ibrutinib have been recorded including hematological toxicity in 7 patients, gastro-intestinal intolerance in 4 patients, arthralgias, atrial fibrillation and asthenia in one patient each.
Conclusion: In indolent clinical forms of MCL frontline ibrutinib in combination with rituximab has a high efficacy, including undetectable MRD in the majority of cases, with a predictable toxicity profile.
Gine:Roche: Other: Travel expenses, Research Funding; Gilead: Other: Travel expenses, Research Funding; Janssen: Other: Travel expenses, Research Funding. Martín:Gilead: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Teva: Research Funding; Roche: Consultancy, Honoraria, Other: Travel Expenses; Servier: Honoraria, Other: Travel Expenses; iQone: Consultancy; Janssen: Honoraria, Other: Travel Expenses, Research Funding; Kiowa Kirin: Consultancy. Terol:Janssen: Consultancy, Research Funding; Astra Zeneca: Consultancy; Roche: Consultancy; Gilead: Research Funding; Abbvie: Consultancy. González-Barca:AbbVie: Consultancy, Honoraria; Celgene: Consultancy; Janssen: Consultancy, Honoraria; Kiowa: Consultancy; Roche: Consultancy, Honoraria; Celtrion: Consultancy; Takeda: Honoraria. Lopez-Guillermo:Gilead: Consultancy, Research Funding; Janssen: Research Funding; Roche: Consultancy, Research Funding; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal