Introduction: Advances in the understanding of disease biology, the introduction of new drugs, and better supportive care have improved outcomes in multiple myeloma (MM). Most improvements have been observed in clinical trial and tertiary referral center populations but questions remain about the generalizability of these findings to patients treated in the community.
Methods: We studied all patients diagnosed with MM between 01/01/2001 and 12/31/2015 who had complete demographic and overall survival (OS) data available and were seen at Mayo Clinic (MAYO) or followed in the Surveillance, Epidemiology, and End Results Program (SEER, 18 registry data 2000 - 2016, 11/2018 submission). We retrieved age at diagnosis, sex, date of diagnosis, date of last follow-up, and OS for all patients. OS was defined as the time from diagnosis to death from any cause. Patients who were alive at the end of follow-up (12/31/ 2016) were censored. OS estimates were calculated using the Kaplan-Meier method. Age- and sex-adjusted Cox models were used to assess the association between the 5-year interval of diagnosis and OS. Expected OS estimates were calculated based on United States general population rate tables (Human Mortality Database) using a conditional approach. Standardized mortality ratios (SMR) were calculated by dividing the number of observed deaths by the number of expected deaths in age- and sex-matched general United States population controls. Linear regression models were fit to test for linear trends in early mortality and SMR over time (per 5-year interval). P-values below 0.05 were considered statistically significant.
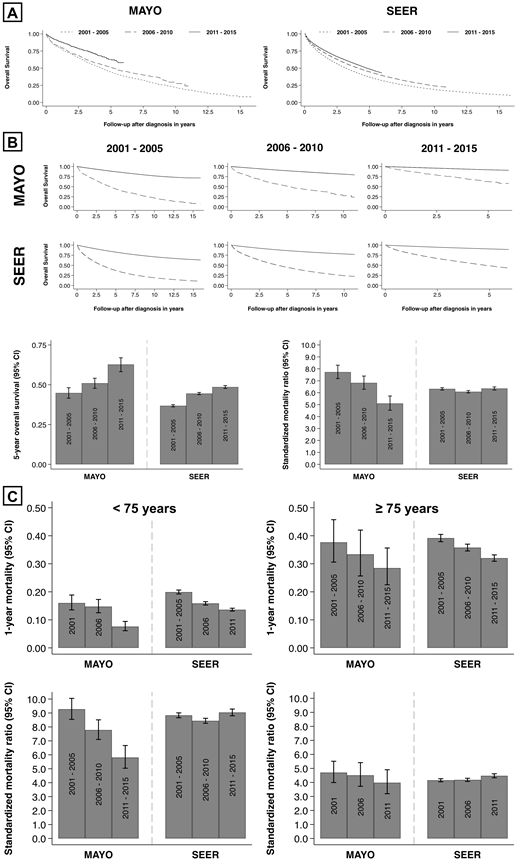
Results: The median age at diagnosis was 3 years lower in patients at MAYO (64 years, 15% ≥ 75 years, 60% male, n = 3293) compared to SEER (67 years, 29% ≥ 75 years, 55% male, n = 61779). After a median follow-up of 2.8 years the median OS was longer in MAYO compared to SEER (5.4 versus 4.0 years, HR 0.82, 95% CI 0.78 - 0.86, p < 0.001) and remained statistically significant after adjusting for age and sex (HR 0.91, 95% CI 0.86 - 0.95, p < 0.001). Early mortality (1-year mortality) decreased between 2001-2005 and 2011-2015: From 20% to 11% at MAYO and from 26% to 19% in SEER. When grouping OS by year of diagnosis (in 5-year-intervals) improvements were seen in both populations (A) and remained statistically significant after adjusting for age and sex. The relative improvements were similar comparing the 5-year period after the introduction of the novel therapies (2006 - 2010) to 2001 - 2005 and more pronounced in MAYO for the most recent 5-year interval (2011 - 2015, A). This trend was reflected in a steady temporal improvement in 5-year OS estimates in MAYO including the most recent 5-year interval (2011 - 2015, B bottom left). In SEER there was a comparable increase between the first two 5-year intervals but a lesser improvement in more recently (2011 - 2015, B bottom left). A diagnosis of MM remained associated with significant excess mortality in all age groups over time in both populations (B top). There was a decrease in excess mortality over time at MAYO (SMR decline per 5-year interval 1.3, 95% CI 0.9 - 1.8, p < 0.001) while there was little change in SEER (SMR decline 0.0, 95% CI -0.3 - 0.3, p = 0.917, B bottom right). Further stratifying by age at diagnosis, the decrease in excess mortality was observed mostly in patients < 75 years at MAYO (SMR decline per 5-year interval 1.7, 95% CI 1.5 - 2.0, p < 0.001, C bottom left) and to a lesser extent in older patients (SMR decline 0.4, 95% CI 0.2 - 0.6, p < 0.001, C bottom right). No such trends towards improvement were observed in either age group in SEER (C bottom). In older patients, early mortality remained approximately 30% in both populations despite continued improvements, while the 5-year OS estimates for the most recent 5-year interval (2011 - 2015) were 37% at MAYO and 26% in SEER (C top).
Conclusions: Both early mortality and long-term survival have improved over time. Reductions in excess mortality were largely confined to younger patients with access to specialized care. Patients ≥ 75 years represent more than a quarter of all patients in the community, a third of them died within one year of the diagnosis, and only one in four was alive five years later. Older patients with MM remain a vulnerable population and have derived only limited benefit from recent advances in the field. Safe and effective therapies for older patients with MM remain an unmet need.
Gertz:Ionis/Akcea: Consultancy; International Waldenstrom Foundation: Research Funding; Alnylam: Consultancy; Prothena Biosciences Inc: Consultancy; Celgene: Consultancy; Janssen: Consultancy; Spectrum: Consultancy, Research Funding; Annexon: Consultancy; Appellis: Consultancy; Amgen: Consultancy; Medscape: Consultancy, Speakers Bureau; Physicians Education Resource: Consultancy; Abbvie: Other: personal fees for Data Safety Monitoring board; Research to Practice: Consultancy; Teva: Speakers Bureau; Johnson and Johnson: Speakers Bureau; DAVA oncology: Speakers Bureau; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Proclara: Membership on an entity's Board of Directors or advisory committees; i3Health: Other: Development of educational programs and materials; Springer Publishing: Patents & Royalties; Amyloidosis Foundation: Research Funding. Dispenzieri:Alnylam: Research Funding; Akcea: Consultancy; Takeda: Research Funding; Pfizer: Research Funding; Janssen: Consultancy; Intellia: Consultancy; Celgene: Research Funding. Lacy:Celgene: Research Funding. Maurer:Nanostring: Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding. Dingli:alexion: Consultancy; Janssen: Consultancy; Millenium: Consultancy; Rigel: Consultancy; Karyopharm: Research Funding. Kapoor:Takeda: Honoraria, Research Funding; Celgene: Honoraria; Janssen: Research Funding; Amgen: Research Funding; Sanofi: Consultancy, Research Funding; Glaxo Smith Kline: Research Funding; Cellectar: Consultancy. Leung:Takeda: Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees; Aduro: Membership on an entity's Board of Directors or advisory committees; Omeros: Research Funding. Kumar:Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal