
Introduction:18F-fluorodeoxyglucose positron emission tomography + computed tomography (FDG-PET/CT) is a reliable imaging technique for staging/monitoring in multiple myeloma (MM) with prognostic value for progression-free survival (PFS). The phase 3 CASSIOPEIA trial investigated the efficacy of CD38 mAb daratumumab + standard-of-care regimen bortezomib/thalidomide/dexamethasone (D-VTd) in TE NDMM patients (pts) vs VTd. In Part 1, D-VTd significantly reduced the risk of disease progression/death and improved stringent complete response (sCR), ≥CR, and minimal residual disease (MRD)-negativity rates vs VTd. Although MRD-negativity is associated with improved outcomes, relapse still occurs in MRD negative pts, potentially due to the presence of focal bone disease. The IFM 2009 trial showed pts with no residual disease assessed by MRD (multiparametric flow cytometry [MFC]) and PET/CT (double negative), achieved better PFS vs pts who were not double negative. Here, we report results of the CASSIOPET companion study of CASSIOPEIA, evaluating the prognostic value of PET/CT at diagnosis, post-consolidation PET-complete response (PET-CR) rates of D-VTd vs VTd, and concordance of PET-CR and MRD negativity.
Methods: In Part 1 of CASSIOPEIA, 1085 TE NDMM pts were randomized to 4 cycles of pre-autologous stem cell transplant (ASCT) induction and 2 cycles of post-ASCT consolidation with D-VTd or VTd. Evaluations of MRD by 8-color MFC (10-5 sensitivity threshold) on bone marrow aspirates were performed and results were correlated with imaging data for the PET/MRD endpoint of CASSIOPET. The primary objective is to compare PFS in pts with MRD negativity and PET-CR post-consolidation vs pts who were not double negative, to be evaluated upon availability of mature PFS data. This analysis focuses on the prognostic value of PET/CT at diagnosis (PFS), post-consolidation double-negativity rates (MRD and PET), and concordance between MRD (MFC) and PET-CR negativity.
Pts were excluded if they were randomized but not treated in CASSIOPEIA (n = 2). PET/CT scans were performed at baseline (prior to first dose) and post-consolidation (Day 100 post-ASCT). Acquired imaging data was uploaded to a central electronic repository system. Per Italian Myeloma criteria for PET Use (IMPeTUs) criteria, 5-point Deauville scores (range, 1-5) were applied to bone marrow (BM), focal lesions (FL), extramedullary disease (EMD), and paramedullary disease (PMD). For each PET dataset, localization of most intense FDG uptake was identified and maximum standardized uptake value (SUVmax) was calculated. CR = lesion uptake < mediastinum blood pool (MBP); unconfirmed CR (uCR) = lesion uptake between MBP and liver. An independent team of nuclear medicine physicians with extensive experience in MM interpreted the images, blinded to pt treatment.
Results: A total of 268 (D-VTd, 137; VTd, 131) pts had assessable baseline PET prior to first dose and 184 (D-VTd, 101; VTd, 83) pts were PET evaluable post-consolidation (pts remaining on study with assessable PET at post-consolidation, baseline PET negative excluded).
At baseline, 20.1% pts were PET negative and 79.9% pts were PET positive. 67.2% pts had FLs (91.7% with uptake >liver), with median SUVmax of 6.115 (range, 1.90-48.50). 22.0% pts had diffuse BM infiltration, and median BM SUVmax was 3.195 (range, 1.44-271.00). PET/CT showed PMD in 17.5% pts, with an PMD SUVmax of 7.110 (range, 1.91-39.80) and EMD in 7.8% of pts, with an EMD SUVmax of 6.850 (range, 3.86-21.14).
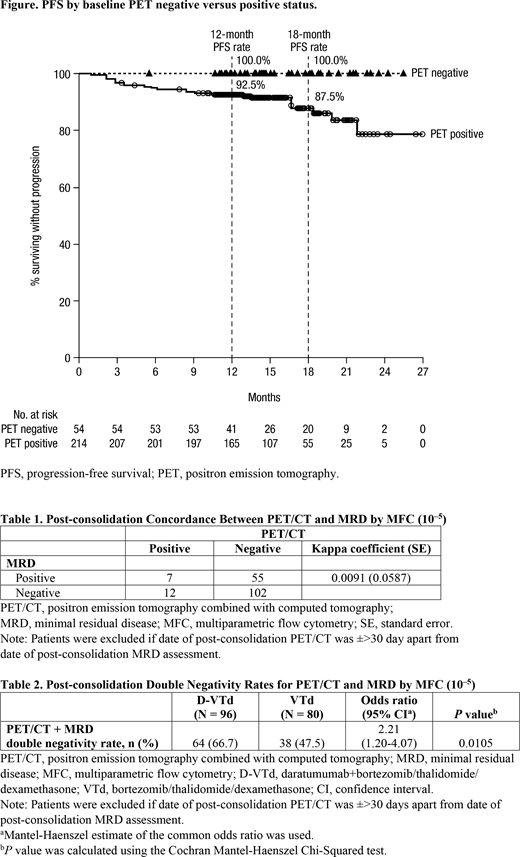
Of 184 pts with post-consolidation PET measurements, 118 (64.1%) pts achieved CR, with 47 (25.5%) pts achieving uCR, 17 (9.2%) with partial response, and 2 (1.1%) pts with stable disease by PET. 12-mo and 18-mo PFS rates in PET-negative vs positive pts at baseline were 100.0% vs 92.5% and 100.0% vs 87.5%, respectively (Figure).
In an assessment of post-consolidation concordance of PET-CR and MRD, 102 pts were MRD and PET-CT negative (Table 1). Post-consolidation double negativity rates of PET/CT and MRD were only 47.5% and 66.7% for VTd vs D-VTd (Table 2).
Conclusions: In CASSIOPET, the PET/CT companion study of the CASSIOPEIA trial, we show that baseline PET-CT findings have prognostic value. More D-VTd pts reach double negativity of PET/CT and MRD post-consolidation vs VTd. With more mature data, PET-CR/MRD negativity concordance may provide insight into utilizing both methods as a predictive surrogate for pt outcomes.
Moreau:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Zweegman:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding. Perrot:Sanofi: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; jannsen: Honoraria, Membership on an entity's Board of Directors or advisory committees; takeda: Honoraria; Amgen: Honoraria. Hulin:celgene: Consultancy, Honoraria; Janssen, AbbVie, Celgene, Amgen: Honoraria. Facon:Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Leleu:Sanofi: Honoraria; Takeda: Honoraria; Oncopeptide: Honoraria; Karyopharm: Honoraria; Amgen: Honoraria; Carsgen: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Janssen: Honoraria; BMS: Honoraria; Merck: Honoraria. Belhadj:Amgen: Honoraria; Celgene: Honoraria, Other: personal fees from Celgene, personal fees from Amgen, personal fees from Takeda, personal fees from Janssen, outside the submitted work; Takeda: Honoraria; Janssen: Honoraria. Karlin:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; AMGEN: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support. Levin:Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Educational Grant; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Educational Grant; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Educational Grant; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Educational grant ; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Educational grant . Minnema:Servier: Honoraria; Amgen: Honoraria; Celgene Corporation: Honoraria, Research Funding; Gilead: Honoraria; Jansen Cilag: Honoraria. Sonneveld:Amgen: Honoraria, Research Funding; BMS: Honoraria; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; SkylineDx: Research Funding. Pei:Janssen: Employment, Equity Ownership. de Boer:Janssen: Employment, Equity Ownership. Vermeulen:Janssen R&D, LLC: Employment, Equity Ownership. Kampfenkel:Janssen: Employment, Equity Ownership.
Bortezomib/thalidomide/dexamethasone (VTd) + autologous stem cell transplantation (ASCT) is standard of care in Europe for transplant-eligible patients with newly diagnosed multiple myeloma. The phase 3 CASSIOPEIA trial evaluated the efficacy and safety of adding daratumumab to VTd (D-VTd) before and after ASCT. Although daratumumab is approved as monotherapy and in combination with standard-of-care regimens for the treatment of MM, D-VTd has not yet been approved. This presentation/paper includes information/discussion of D-VTd in the CASSIOPET companion study to CASSIOPEIA.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal