Introduction:TP53 gene mutations (mTP53), found in up to 20% of MDS or AML pts and 30-40% of therapy-related (TR) MDS/AML cases, represent a distinct molecular cohort with poor outcomes. Hypomethylating agents (HMA) are the standard of care with CR rates of ~20% and median OS of 7-8 months. APR-246 is a novel, first-in-class small molecule that selectively induces apoptosis in mTP53 cancer cells via thermodynamic stabilization of the p53 protein and shifting equilibrium toward the wild-type conformation. We previously reported the Phase 1b results of APR-246+AZA with no DLTs, transcriptional activation of p53 targets and high response rates, identifying a Phase 2 (P2) dose of 4500mg days 1-4 (Sallman et al., ASH 2018). We report herein the planned, completed phase 2 results.
Methods: This is a multicenter Phase 1b/2 trial of APR-246+AZA in HMA-naïve mTP53 higher risk MDS, MDS/MPN and oligoblastic AML (≤ 30% blasts) pts (NCT03072043). P2 pts received APR-246 4500mg IV (days 1-4) + AZA 75 mg/m2 SC/IV x 7 days (days 4-10 or 4-5 and 8-12) in 28 day cycles. Primary objective was CR rate by International Working Group (IWG) 2006 criteria. Secondary objectives included ORR, OS, outcome following allogeneic hematopoietic stem cell transplant (allo-HSCT), and both next generation sequencing (NGS) and p53 immunohistochemistry (IHC) to monitor clonal suppression and remission depth as prognostic covariates. For minimal residual disease (MRD) analysis, a custom target-capture NGS assay was developed using unique molecular Identifiers for error correction with a 0.1% limit of detection.
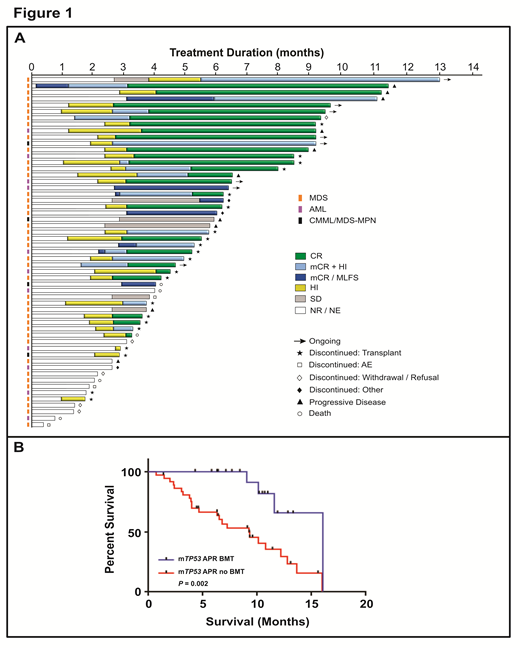
Results: As of July 15, 2019, 55 pts were enrolled (6 P1; 49 P2) with a median age 66 years (34-85; 47% male). By WHO, 40 pts had MDS, 11 AML-MRC and 4 CMML/MDS-MPN; 85% had complex cytogenetics and 33% TR-MDS/AML. All pts had higher risk disease by IPSS-R (7% Intermediate, 24% High, 69% Very High). Fifty pts (91%) had a TP53 missense mutation in the DNA binding domain with multiple mutations in 18 (33%), and median variant allele frequency (VAF) of 25%. In 34 pts (62%), TP53 was the sole mutation. Median time on treatment is 154 days (11-392) with 8 pts ongoing. Eighteen pts (33%; 40% of evaluable pts) discontinued study treatment to proceed to allo-HSCT. Treatment (Tx)-related AEs in ≥ 20% of pts included nausea/vomiting (58%), dizziness (31%), constipation (24%), neuropathy (22%), leukopenia (22%) and thrombocytopenia (20%; all G1/G2 except cytopenias (G3/G4). Tx-related febrile neutropenia and anemia occurred in 9% and 5% of pts with no other G3/G4 event in >1 pt. Thirty and 60 day mortality was 2% (n=1) and 6% (n=3), respectively.
At data cutoff, 45pts were response evaluable with a median follow up of 10.5 months (Fig 1A). ORR by IWG was 87% (39/45) with 24 CR (53%), 8 marrow CR (mCR)+HI (18%), 3 HI alone (7%), and 4 with mCR (9%). Of 6 non-responders, 4 had stable disease and 2 pts had progressive disease. Median time to response was 2.1 months (0.1-5.4) and median duration of response of 6.5 months. CR rate for MDS was 61% (20/33), 50% for AML (4/8) and 0% for MDS/MPN (0/4) with an 88% ORR rate for MDS/AML and 75% for MDS/MPN. An isolated mTP53 was predictive for a higher CR rate (69% vs 25%; P=.006) with a trend for higher ORR (93% vs 75%; P=.17). Additionally, pts with >10% p53 IHC+ BM-MNC was a covariate associated with higher CR rate (66% vs 13%; P=.01). Complete and partial cytogenetic response occurred in 41% (n=18) and 18% (n=8) of pts, respectively. On serial TP53 NGS using a VAF cutoff of 5%, 39% (n=21) of patients achieved NGS negativity, which was associated with improved OS (12.8 vs 9.2 months; P=.02). In NGS- pts, the median MRD VAF at maximum clearance was 0.63% (0.0%-5%) with 5 pts (11%) MRD negative. By intention-to-treat analysis, median OS was 11.6 months (95% CI 9.2-14) with significantly longer OS in responding pts (12.8 vs 3.9 months; P<.0001). Pts undergoing allo-HSCT had improved median OS (16.1 [95% CI 11.6-NE] vs 9.2 [95% CI 6.3-13.7] months), with a 1-year OS of 66% vs 29% in pts who were not transplanted (P=.002; Fig 1B). All NGS- pts prior to allo-HSCT remain alive at date cutoff.
Conclusions: APR-246+AZA is a well-tolerated combination with high response rates in mTP53 MDS/AML. Response durations are promising accompanied by a high fraction of cytogenetic and deep molecular remissions leading to encouraging outcomes post-HSCT. These data support the ongoing, randomized phase 3 study of APR-246+AZA versus AZA alone in mTP53 MDS (NCT03745716).
Sallman:Abbvie: Speakers Bureau; Novartis: Speakers Bureau; Jazz: Research Funding; Incyte: Speakers Bureau; Celyad: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding, Speakers Bureau. DeZern:Astex Pharmaceuticals, Inc.: Consultancy; Celgene: Consultancy. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Steensma:Stemline: Consultancy; Pfizer: Consultancy; Aprea: Research Funding; H3 Biosciences: Other: Research funding to institution, not investigator.; Astex: Consultancy; Arrowhead: Equity Ownership; Onconova: Consultancy; Summer Road: Consultancy. Roboz:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Actinium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amphivena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eisai: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees; Otsuka: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trovagene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sekeres:Syros: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Cluzeau:Jazz Pharma: Consultancy; Abbvie: Consultancy; Menarini: Consultancy. Sweet:Incyte: Research Funding; Celgene: Speakers Bureau; Pfizer: Consultancy; Agios: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Jazz: Speakers Bureau; Stemline: Consultancy; Abbvie: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Korbel:Aprea Therapeutics: Employment. Attar:Aprea Therapeutics: Employment. Kantarjian:Astex: Research Funding; Amgen: Honoraria, Research Funding; Ariad: Research Funding; BMS: Research Funding; AbbVie: Honoraria, Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Immunogen: Research Funding; Jazz Pharma: Research Funding; Takeda: Honoraria; Pfizer: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Agios: Honoraria, Research Funding; Cyclacel: Research Funding. Lancet:Daiichi Sankyo: Consultancy, Other: fees for non-CME/CE services ; Agios, Biopath, Biosight, Boehringer Inglheim, Celator, Celgene, Janssen, Jazz Pharmaceuticals, Karyopharm, Novartis: Consultancy; Pfizer: Consultancy, Research Funding. Fenaux:Aprea: Research Funding; Astex: Honoraria, Research Funding; Celgene Corporation: Honoraria, Research Funding; Jazz: Honoraria, Research Funding. List:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Komrokji:JAZZ: Consultancy; Novartis: Speakers Bureau; Incyte: Consultancy; DSI: Consultancy; pfizer: Consultancy; celgene: Consultancy; JAZZ: Speakers Bureau; Agios: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal