Background: PD1 inhibitor (PD1i) efficacy is taken as evidence of T cell exhaustion in Classical Hodgkin Lymphoma (CHL), however, direct evidence for exhaustion is limited. PD1 expression does not predict PD1i response in CHL. Additionally, PDL1 and PD1 expression are not correlated and are independently prognostic. This suggests a more complex picture than PD1 expression simply reflecting exhaustion induced by PDL1.
Aim: To investigate PD1/PDL1 interaction in CHL
Methods: 149 CHL cases were compared to 27 reactive lymph node controls (RLN) by immunohistochemistry (IHC). PD1 expression was validated with two clones. Multiplex IHC (mIHC) was performed in 47 CHL and 27 RLN by stripping, reprobing and image alignment. Imaging Mass Cytometry (IMC) was analysed by t-SNE clustering and spatial point pattern modelling (SPPM). SPPM is a powerful technique adopted from other disciplines and its use in multiplex image analysis is novel. Flow-based CFSE proliferation and cytokine assays were performed in 15 CHL and 5 RLN single cell suspensions (SCS) with CD3/28 and PMA/Ionomycin stimulation. Co-cultures were with KMH2 and L428 cell lines and healthy donor naïve T helper cells (TH).
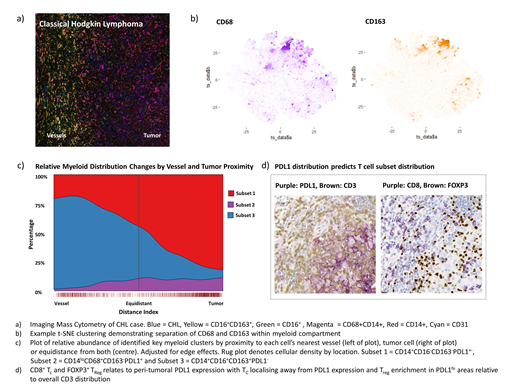
Results: We evaluated PDL1 expression by IHC and found that PDL1hi CHL cells were surrounded by PDL1+ macrophages (Mφ), consistent with the literature. Mφ PDL1 intensity correlated inversely with distance from CHL cells consistent with induction by a CHL-secreted factor (p <0.001), a finding we validated in vitro (p <0.001). Deep phenotyping using IMC identified CD14+CD16-CD163-PDL1+-, CD14-CD68+CD163-PDL1+ and CD14+CD16+CD163+PDL1- myeloid subsets by t-SNE. SPPM revealed that tumor and vessel location predicted their distribution with subsets one and two localising to tumor whilst subset three localised to vessel. Our data supports the hypothesis that CHL recruits and induces a PDL1+ myeloid environment.
We next sought evidence of T cell exhaustion. PD1 expression by IHC was lower in CHL than RLN (p<0.001). Cells co-expressing PD1 with TIM3 or LAG3 were significantly reduced in CHL compared to RLN by mIHC. Cells co-expressing PD1 with TBET or EOMES were reduced in or showed no significant difference between CHL and RLN. No correlation was seen between PD1 and PDL1 expression. We performed functional validation, evaluating proliferative and IL2/IFNγ production capacity, and identified no significant difference between CHL and RLN SCS with or without the addition of Pembrolizumab or isotype control. This constitutes a more detailed assessment of exhaustion with higher case numbers than publications to date. Our data does not support the presence of an exhausted T population above that seen in reactive controls and finds no relationship between PD1 and PDL1 expression. The absence of exhaustion does not exclude a role for the PD1-PDL1 axis, which regulates multiple facets of T cell homeostasis including motility, differentiation (skewing towards T regulatory cells (TReg)), and TReg effector functions. Many of these are characterised by transient PD1 expression unlike the sustained expression seen in exhaustion.
In contrast to PD1, CHL PDL1 and MHC class 2 expression do predict PD1i response. CHL MHC2 expression suggests antigen presentation and a role for TH cells. This led us to examine connections between PDL1 and the TH compartment. Co-culture of CHL cell lines with naïve TH led to enrichment of CD25hiCD127loFOXP3+ TReg which upregulated PD1 compared to TReg from paired CD3/28 controls (p<0.001). FOXP3+ cells were enriched by IHC compared to RLN (p<0.001). Unlike PD1, FOXP3 associated with CHL MHC2 expression (p=0.012), which in turn associated with PDL1 expression (p=0.02). SPPM of TC and TH subsets revealed that PDL1 expression predicted the location of FOXP3+ TReg and negatively predicted the location of TC and TH17. PDL1 was a stronger predictor than CHL cell density. These data support a role for PDL1 in shaping T differentiation and trafficking irrespective of observed PD1 expression.
Conclusion: We find that CHL cells are PDL1hi and induce a PDL1+ myeloid environment, but detect little evidence of T exhaustion. Instead we find that PDL1 expression shapes T (particularly TReg) differentiation and trafficking. Our data challenges the narrative that PD1i in CHL act by reversing exhaustion and highlights novel mechanisms with the potential to provide insight into CHL biology and inform the hunt for biomarkers of response.
Gribben:Acerta/Astra Zeneca: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal